This is a preprint.
Altered striatal dopamine regulation in ADGRL3 knockout mice
- PMID: 40766670
- PMCID: PMC12324523
- DOI: 10.1101/2025.07.31.667389
Altered striatal dopamine regulation in ADGRL3 knockout mice
Abstract
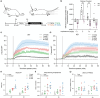
Dopaminergic signaling is essential for regulating movement, learning, and reward. Disruptions in this system are linked to neuropsychiatric disorders such as ADHD. ADGRL3, an adhesion G protein-coupled receptor highly expressed in the brain, is genetically associated with increased ADHD risk. ADGRL3 knockout in animals alters expression of dopaminergic markers and induces dopamine-related behavioral changes. However, its precise role in modulating dopamine signaling remains unclear. We investigated how ADGRL3 knockout affects striatal dopamine release in mice using ex vivo fast-scan cyclic voltammetry and in vivo fiber photometry with a dopamine sensor. Ex vivo measurements showed increased electrically-evoked dopamine release across the striatum. Conversely, in vivo recordings revealed reduced task-induced dopamine signals in the nucleus accumbens during an operant fixed interval task. This reduction was not due to impaired dopamine availability, as amphetamine-evoked release was unchanged. These findings suggest ADGRL3 modulates dopamine release in complex ways via different pre- and postsynaptic mechanisms.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures






Similar articles
-
adgrl3.1 knockout disrupts cortisol regulation and stress reactivity, linking externalizing and internalizing behaviors.Behav Brain Res. 2025 Oct 2;494:115727. doi: 10.1016/j.bbr.2025.115727. Epub 2025 Jul 8. Behav Brain Res. 2025. PMID: 40639688
-
High ovarian hormones present during fear extinction reduce fear relapse through a nigrostriatal dopamine pathway.Biol Sex Differ. 2025 Jun 1;16(1):38. doi: 10.1186/s13293-025-00722-7. Biol Sex Differ. 2025. PMID: 40452046 Free PMC article.
-
Preclinical and clinical sex differences in the effects of alcohol on measures of brain dopamine: a systematic review.Biol Sex Differ. 2025 Apr 8;16(1):24. doi: 10.1186/s13293-025-00706-7. Biol Sex Differ. 2025. PMID: 40200334 Free PMC article.
-
Nucleus accumbens dopamine encodes the trace period during appetitive Pavlovian conditioning.bioRxiv [Preprint]. 2025 Apr 3:2025.01.07.631806. doi: 10.1101/2025.01.07.631806. bioRxiv. 2025. Update in: eNeuro. 2025 May 27;12(5):ENEURO.0016-25.2025. doi: 10.1523/ENEURO.0016-25.2025. PMID: 40235964 Free PMC article. Updated. Preprint.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Arcos-Burgos M., Jain M., Acosta M. T., Shively S., Stanescu H., Wallis D., Domené S., Vélez J. I., Karkera J. D., Balog J., Berg K., Kleta R., Gahl W. A., Roessler E., Long R., Lie J., Pineda D., Londoño A. C., Palacio J. D., Arbelaez A., Lopera F., Elia J., Hakonarson H., Johansson S., Knappskog P. M., Haavik J., Ribases M., Cormand B., Bayes M., Casas M., Ramos-Quiroga J. A., Hervas A., Maher B. S., V Faraone S., Seitz C., Freitag C. M., Palmason H., Meyer J., Romanos M., Walitza S., Hemminger U., Warnke A., Romanos J., Renner T., Jacob C., Lesch K.-P., Swanson J., Vortmeyer A., Bailey-Wilson J. E., Castellanos F. X., Muenke M., A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry 15, 1053–1066 (2010). - PubMed
-
- Arcos-Burgos M., Vélez J. I., Martinez A. F., Ribasés M., Ramos-Quiroga J. A., Sánchez-Mora C., Richarte V., Roncero C., Cormand B., Fernández-Castillo N., Casas M., Lopera F., Pineda D. A., Palacio J. D., Acosta-López J. E., Cervantes-Henriquez M. L., Sánchez-Rojas M. G., Puentes-Rozo P. J., Molina B. S. G., M. T. A. C. Group, Boden M. T., Wallis D., Lidbury B., Newman S., Easteal S., Swanson J., Patel H., Volkow N., Acosta M. T., Castellanos F. X., de Leon J., Mastronardi C. A., Muenke M., ADGRL3 (LPHN3) variants predict substance use disorder. Transl Psychiatry 9, 42 (2019). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
