Inhibitory effects of herbal monomers on ferroptosis in renal fibrosis: a review and mechanistic study
- PMID: 40766752
- PMCID: PMC12321836
- DOI: 10.3389/fphar.2025.1610573
Inhibitory effects of herbal monomers on ferroptosis in renal fibrosis: a review and mechanistic study
Abstract
Background and purpose: Renal fibrosis is a common characteristic of chronic kidney disease (CKD). Studies have confirmed the role of ferroptosis in the pathogenesis of various kidney diseases, making it a new research hotspot in the field of renal fibrosis. Monomers of Chinese herbal medicines (CHMs) can improve renal fibrosis by multi-target inhibition of ferroptosis. This review aimed to explore the roles and mechanisms of CHMs in renal fibrosis.
Methods: Using the keywords "ferroptosis", "chronic kidney disease", "renal fibrosis", "Chinese herbal medicine", "natural products", "bioactive components", and "herb", we conducted an extensive literature search of several databases, including PubMed, Web of Science, CNKI, and Wanfang database, to identify studies reporting the role of CHM monomers in inhibiting ferroptosis and improving renal fibrosis. The names of the plants covered in the review have been checked through MPNS (http://mpns.kew.org). All monomers of CHMs were identified in the Pharmacopoeia of the People's Republic of China.
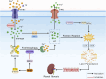
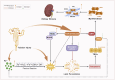
Results: In total, 21 monomers of CHMs were identified in this study, most of which were flavonoids, followed by terpenoids and coumarins. This review showed that monomers of CHMs inhibited ferroptosis and improved renal fibrosis through multi-target mechanisms. They maintained iron homeostasis by acting on NCOA4 and Nrf2 to reduce ferritinophagy. They also inhibited lipid peroxidation and regulated the antioxidant system by modulating ACSL4, NOX4, Nrf2, FSP1, and GPX4 and inhibiting Smad3 to improve renal fibrosis.
Conclusion: Monomers of CHMs effectively inhibited ferroptosis and prevented renal fibrosis in various animal models and cell models of CKD. However, further in-depth studies with better designs are needed to identify the exact targets of monomers of CHMs and improve the treatment of renal fibrosis and CKD.
Keywords: Chinese herbal medicine; chronic kidney disease; ferroptosis; monomers; renal fibrosis.
Copyright © 2025 Liu, Yu, He, Wang, Li, Wang and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

