Molecular mechanism underlying Oleum Cinnamomi-induced ferroptosis in MRSE via covalent modification of AhpC
- PMID: 40766757
- PMCID: PMC12321849
- DOI: 10.3389/fphar.2025.1554294
Molecular mechanism underlying Oleum Cinnamomi-induced ferroptosis in MRSE via covalent modification of AhpC
Abstract
Introduction: Oleum Cinnamomi (OC) is a volatile oil extracted by steam distillation from the dried branches and leaves of Cinnamomum cassia Presl, a plant belonging to the Lauraceae family. For centuries, OC has been utilized as a food preservative and flavoring agent, demonstrating potent inhibitory effects against bacteria and fungi. It is particularly effective in controlling infections caused by Methicillin-Resistant Staphylococcus epidermidis (MRSE), which often parasitizes the skin surface. To uncover the target and molecular mechanism by which OC eradicates MRSE, this study initially assessed the impact of OC and its primary constituents on oxidative stress in MRSE cells.
Methods: Mass spectrometry was employed to identify the target and covalent binding sites of OC, while a kit was used to monitor changes in key biomolecules of MRSE cells exposed to OC. Additionally, the efficacy of OC in inhibiting MRSE adhesion and infection of RAW 264.7 mouse macrophages was evaluated.
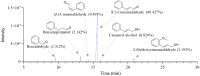
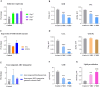
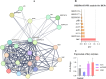
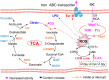
Results: The findings revealed that OC's main components, cinnamaldehyde and 2-methoxycinnamaldehyde, covalently modify MRSE and AhpC. This modification disrupts the AhpC-AhpE regeneration cycle, thereby disturbing both enzymatic and non-enzymatic redox homeostasis. It leads to intracellular ROS accumulation and effectively prevents MRSE from adhering to RAW 264.7 mouse macrophages. In response to ROS detoxification, MRSE attempts to upregulate the expression of TCA cycle-related proteins. However, the continuous accumulation of ROS inactivates the [Fe-S] protein of Aconase (ACO), hindering ACO's catalytic conversion of citric acid to isocitrate. This results in sustained intracellular accumulation of citric acid, limiting the TCA cycle and ATP generation. Simultaneously, enzymes involved in reduction catalysis, such as superoxide dismutase (SOD), peroxidase reductase (Prx), and glutathione synthase (GCL), are collectively inactivated. OC induces oxidative stress in MRSE, depleting GSH and triggering lipid peroxidation, which in turn induces MRSE to undergo ferroptosis.
Discussion: This covalent inhibition strategy targeting AhpC to induce ferroptosis offers a promising approach for effectively treating and preventing MRSE infections, thereby opening new avenues for combating drug-resistant pathogen infections.
Keywords: AhpC; MRSE; Oleum Cinnamomi (OC); ROS; covalent inhibitors; metabolic pathways.
Copyright © 2025 Wang, Wu, Chen, Yan, Ling, He, Jin, Zhao, Peng and Yang.
Conflict of interest statement
Author YL was employed by Guangdong L-Med Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Exploring the additive antibacterial potential of Cinnamomum cassia volatile oil and imipenem against Acinetobacter baumannii: a multi-omics investigation.Front Microbiol. 2025 Jul 2;16:1578322. doi: 10.3389/fmicb.2025.1578322. eCollection 2025. Front Microbiol. 2025. PMID: 40673148 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Downregulation of LATS1/2 Drives Endothelial Senescence-Associated Stemness (SAS) and Atherothrombotic Lesion Formation.bioRxiv [Preprint]. 2025 Jun 21:2025.06.19.660635. doi: 10.1101/2025.06.19.660635. bioRxiv. 2025. PMID: 40667385 Free PMC article. Preprint.
-
Perillaldehyde synergizes with ferroptosis inducers to promote ferroptotic cell death in gastric cancer.Front Cell Dev Biol. 2025 Jun 3;13:1598520. doi: 10.3389/fcell.2025.1598520. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40530331 Free PMC article.
References
-
- Chen J., Zhang J. N., Zhu L. P., Qian C. G., Tian H. R., Zhao Z. M., et al. (2022). Antibacterial activity of the essential oil from Litsea cubeba against Cutibacterium acnes and the investigations of its potential mechanism by gas chromatography-mass spectrometry metabolomics. Front. Microbiol. 13, 823845. 10.3389/fmicb.2022.823845 - DOI - PMC - PubMed
-
- Damasceno R. O. S., Pinheiro J. L. S., da Silva L. D., Rodrigues L. H. M., Emídio J. J., Lima T. C., et al. (2025). Phytochemistry and anti-inflammatory and antioxidant activities of Cinnamomum osmophloeum and its bioactive constituents: a review. Plants-Basel 14 (4), 562. 10.3390/plants14040562 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

