Mucoadhesive Mini-Containers with Unidirectional Drug Release Capacity for Macromolecular Therapeutics
- PMID: 40766820
- PMCID: PMC12323886
- DOI: 10.2147/DDDT.S521900
Mucoadhesive Mini-Containers with Unidirectional Drug Release Capacity for Macromolecular Therapeutics
Abstract
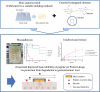
Purpose: Peptide-based therapeutics have gained widespread attention for their high specificity and efficacy. However, their oral delivery remains challenging owing to their poor stability and bioavailability in the gastrointestinal environment and limited membrane permeability. To address these barriers, we have designed a novel mini-container system with unidirectional drug release and enhanced mucoadhesion capacities.
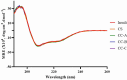
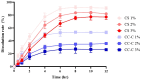
Methods: Mini-containers composed of ethyl cellulose shells of varying degrees of viscosity were fabricated using a simple molding process and integrated with catechol-conjugated chitosan (CC) to improve their mucosal adhesion capacity and structural stability.
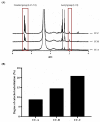
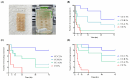
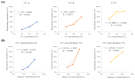
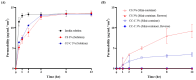
Results: The catechol substitution levels were optimized (CC-A, CC-B, and CC-C), with the CC-C formulation exhibiting the highest degree of substitution (20.93%) and superior adhesion capacity, maintaining 80% attachment on porcine small intestinal mucosa after 72 h. Insulin, a model peptide drug, was successfully loaded into the CC-C mini-containers, and circular dichroism spectroscopy analysis confirmed that its secondary structure remained intact. The insulin content in the mini-containers, as determined by HPLC-UV analysis, demonstrated consistency across formulations: 101.1 ± 2.4% for 1% CC-C, 95.4 ± 3.8% for 2% CC-C, and 100.0 ± 1.8% for 3% CC-C, while in-vitro dissolution and Franz diffusion cell studies demonstrated its sustained and unidirectional release. After 12 hours of dissolution, the 3% CC-C formulation showed a release rate of 26.22 ± 2.23%, while the 1% CC-C formulation exhibited a release rate of 53.11 ± 0.25%. Catechol-mediated crosslinking significantly slowed the release rate relative to that of controls. The robust structure of the mini-containers fabricated with high-viscosity ethyl cellulose exhibited a mechanical strength of 13.21 ± 0.50 N, comparable to that of commercial enteric capsules (10 N), ensuring durability under gastrointestinal conditions.
Conclusion: This study shows the potential of mini-container technology for the stable and prolonged oral delivery of macromolecular therapeutics. However, further investigation is required to confirm its effectiveness in-vivo.
Keywords: catechol-conjugated chitosan; mini-container shell; mold; mucoadhesion; unidirectional.
© 2025 Han et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Cao S-J, Lv Z-Q, Guo S, Jiang G-P, Liu H-L. An update-prolonging the action of protein and peptide drugs. J Drug Delivery Sci Technol. 2021;61:102124 doi: 10.1016/j.jddst.2020.102124. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

