Immunothrombosis and plasma fibrinolytic function for pediatric COVID-19: a secondary analysis from the COVAC-TP trial
- PMID: 40766870
- PMCID: PMC12320441
- DOI: 10.1016/j.bvth.2024.100038
Immunothrombosis and plasma fibrinolytic function for pediatric COVID-19: a secondary analysis from the COVAC-TP trial
Abstract
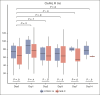
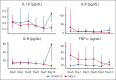
The relationship between fibrinolysis, inflammation, and prothrombotic risk among children hospitalized for coronavirus disease 2019 (COVID-19)-related illness is ill defined. To investigate the association between plasma fibrinolytic capacity and proinflammatory cytokine concentrations among children hospitalized for primary COVID-19 infection and multisystem inflammatory syndrome in children (MIS-C), we hypothesized that cytokine concentrations differ by clinical phenotype and are associated with hypofibrinolysis. We analyzed banked plasma specimens serially collected from children aged <18 years admitted for primary COVID-19 or MIS-C and enrolled in the COVID-19 Anticoagulation in Children-Thromboprophylaxis multicenter trial, an open-label, multicenter, phase 2 clinical trial conducted between July 2020 and May 2021. Plasma coagulative and fibrinolytic function were measured via the clot formation and lysis (CloFAL) assay and modified mini-euglobulin clot lysis assay (ECLA). Interleukin-1β (IL-1β), IL-6, and IL-8, and tumor necrosis factor α were measured by the Meso Scale Discovery assay. Correlations were evaluated using Spearman rank testing. A total of 132 banked plasma specimens from 38 participants (COVID-19: n = 18; MIS-C: n = 20) were analyzed. Overall, increased coagulative function (ie, elevated CloFAL area under the curve) and impaired fibrinolytic function (ie, reduced CloFAL fibrinolytic index [FI] and elevated modified mini-ECLA clot lysis time ratio [CLTR]) were observed but most notably among those with MIS-C. Plasma cytokine concentrations correlated with assay indices of hypofibrinolysis (ie, modified mini-ECLA CLTR and CloFAL FI). In summary, among children hospitalized for COVID-19-related illness, hypercoagulability and hypofibrinolysis are mediated, in part, by inflammation that may contribute to prothrombotic risk. This trial was registered at www.ClinicalTrials.gov as #NCT04354155.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests. A complete list of the members of the COVID-19 Anticoagulation in Children–Thromboprophylaxis Trial Investigators appears in the supplemental Appendix.
Figures





Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children.Cochrane Database Syst Rev. 2015 Oct 14;2015(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. Cochrane Database Syst Rev. 2015. PMID: 26465274 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Safety and efficacy of repeat ivermectin mass drug administrations for malaria control (RIMDAMAL II): a phase 3, double-blind, placebo-controlled, cluster-randomised, parallel-group trial.Lancet Infect Dis. 2025 Jul;25(7):737-750. doi: 10.1016/S1473-3099(24)00751-5. Epub 2025 Feb 4. Lancet Infect Dis. 2025. PMID: 39919778 Clinical Trial.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
