Long-Term Memory Engrams From Development to Adulthood
- PMID: 40767385
- PMCID: PMC12326896
- DOI: 10.1002/hipo.70032
Long-Term Memory Engrams From Development to Adulthood
Abstract
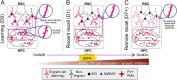
Memories formed in adulthood can last a lifetime, whereas those formed early in life are rapidly forgotten through a process known as infantile amnesia. In recent years, tremendous progress has been made in understanding the memory engram-the physical trace of a memory in the brain-and how it transforms as memories evolve from recent to remote. This review focuses on engram cells and examines their roles in memory encoding, consolidation, retrieval, and forgetting from development to adulthood. We concentrate on four key brain regions: the hippocampus, the retrosplenial cortex, the medial prefrontal cortex, and the anterior thalamic nuclei. We first focus on the adult brain, highlighting recent studies that reveal the distinct contributions of engram cells in each brain region, with particular emphasis on synaptic plasticity and memory consolidation. We then explore how coordinated activity across these regions supports long-term memory. In the second section, we review emerging knowledge of engram cells in the developing brain, examining how developmental differences in their functions contribute to infant memory generalization and infantile amnesia. Compared to adults, much less is known about how, or to what extent, early-life memories undergo consolidation. In the final section, we synthesize current knowledge of memory consolidation and retrieval in the adult brain with what is known about the development of the four brain regions we discuss. We then propose key directions for future research. In sum, this review brings together recent findings that deepen our understanding of the dynamic changes in memory engrams that underlie consolidation and long-term storage and explores how developmental differences may shape the maturation of memory processes.
Keywords: development; infantile amnesia; memory consolidation; memory engram; synaptic plasticity.
© 2025 The Author(s). Hippocampus published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Amnesia after Repeated Head Impact Is Caused by Impaired Synaptic Plasticity in the Memory Engram.J Neurosci. 2024 Feb 21;44(8):e1560232024. doi: 10.1523/JNEUROSCI.1560-23.2024. J Neurosci. 2024. PMID: 38228367 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Arai, M. , Osanai H., Snell C. C., Kitamura T., and Ogawa S. K.. 2025. “Combinative Protein Expression of Immediate Early Genes c‐Fos, Arc, and Npas4 Along Aversive‐ and Reward‐Related Neural Networks.” BioRxiv 2025.04.21.649441 10.1101/2025.04.21.649441. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

