Progression Patterns in Foveal-Sparing Geographic Atrophy With Double-Layer Sign Due to Neovascularization or Basal Laminar Deposits
- PMID: 40767440
- PMCID: PMC12347187
- DOI: 10.1167/iovs.66.11.16
Progression Patterns in Foveal-Sparing Geographic Atrophy With Double-Layer Sign Due to Neovascularization or Basal Laminar Deposits
Abstract
Purpose: The purpose of this study was to analyze the prognostic significance of double-layer sign (DLS) in eyes with foveal-sparing geographic atrophy (GA) secondary to age-related macular degeneration.
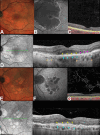
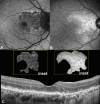
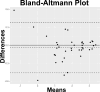
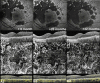
Methods: This retrospective, observational cohort study analyzed 46 eyes (46 patients) with foveal sparing GA and associated DLS, using fundus autofluorescence (FAF), near-infrared reflectance (NIR), optical coherence tomography (OCT), and OCT angiography (OCTA). DLS was defined based on OCTA findings as either thick basal laminar deposits (BLamD) or non-exudative macular neovascularization (NE-MNV). The area of GA and foveal sparing were estimated on both FAF and NIR at different time points. Centrifugal and centripetal GA growth rates referring to the lesion expansion away from and toward the fovea, respectively, were evaluated using a mathematical formula.
Results: Of the 46 eyes enrolled, 25 had thick BLamD, whereas 21 had type 1 NE-MNV. The NE-MNV eyes showed significantly thicker DLS than those with BLamD (90.4 ± 39.8 µm vs. 57.0 ± 27 µm, 95% confidence interval [CI] = 0.34 to 0.78, P < 0.001). GA areas were smaller on FAF than NIR (95% CI = -0.89 to -0.03, P = 0.03) in the BLamD group, whereas no difference was observed in the NE-MNV group (95% CI = -0.37 to 0.64, P = 0.60). Despite similar GA areas, the NE-MNV eyes exhibited larger foveal sparing (95% CI = 0.02 to 1.21, P = 0.04). The foveal sparing area remained stable (F(1.2, 11 = 4.15, P = 0.06, ω2 = 0.02) in the NE-MNV group, whereas a significant reduction was observed in the BLamD subgroup (F(1.39, 20.9) = 7.5, P < 0.001, ω2 = 0.09).
Conclusions: OCTA has provided valuable insights into the pathogenic interpretation of the DLS signature. Our findings confirm that a neovascular DLS protects the retinal pigment epithelium and outer retina, contributing to prolonged foveal preservation.
Conflict of interest statement
Disclosure:
Figures
Similar articles
-
A Brazilian case of extensive macular atrophy with pseudodrusen and non-exudative quiescent macular neovascularization.Eur J Ophthalmol. 2025 Jul;35(4):NP44-NP48. doi: 10.1177/11206721251333274. Epub 2025 Apr 1. Eur J Ophthalmol. 2025. PMID: 40170431
-
Clinical and imaging characteristics associated with foveal neovascularization in proliferative diabetic retinopathy.Graefes Arch Clin Exp Ophthalmol. 2025 Mar;263(3):679-687. doi: 10.1007/s00417-024-06660-1. Epub 2024 Nov 15. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 39542876
-
Determinants of Four-Year Visual Acuity Loss in Geographic Atrophy: An Analysis of Age-Related Eye Disease Study and Age-Related Eye Disease Study 2.Ophthalmology. 2025 Jul;132(7):785-798. doi: 10.1016/j.ophtha.2025.01.028. Epub 2025 Feb 5. Ophthalmology. 2025. PMID: 39921001 Clinical Trial.
-
Progression Rate of Macular Retinal Pigment Epithelium Atrophy in Geographic Atrophy and Selected Inherited Retinal Dystrophies. A Systematic Review and Meta-Analysis.Am J Ophthalmol. 2025 Jan;269:30-48. doi: 10.1016/j.ajo.2024.07.035. Epub 2024 Aug 15. Am J Ophthalmol. 2025. PMID: 39153684
-
Diagnostic accuracy of OCT angiography for macular neovascularization in central serous chorioretinopathy: A systematic review and meta-analysis.Acta Ophthalmol. 2024 Nov;102(7):749-758. doi: 10.1111/aos.16739. Epub 2024 Jul 11. Acta Ophthalmol. 2024. PMID: 38988251
References
-
- Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. - PubMed
-
- Sarks JP, Sarks SH, Killingsworth MC.. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988; 2(Pt 5): 552–577. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

