In Vivo Cone Photoreceptor Topography of the Human Foveola
- PMID: 40767443
- PMCID: PMC12347159
- DOI: 10.1167/iovs.66.11.13
In Vivo Cone Photoreceptor Topography of the Human Foveola
Abstract
Purpose: To study in vivo cone topography of the normal human foveola.
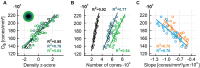
Methods: The fovea in both eyes of 30 healthy participants was imaged with adaptive optics scanning light ophthalmoscopy. High-resolution image montages spanning two degrees of visual angle were created and cone center locations annotated. Continuous cone density maps were computed by a Voronoi cell area approach to also yield the topographical center, the cone density centroid (CDC). Cone density profiles were extracted and fit with a four-parameter decay function, D = D0 / (1 + (E/a)b)c, with D as cone density (cones/mm2), D0 as cone density at the CDC, and E as eccentricity (µm).
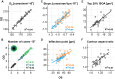
Results: Across eyes, D0 was 175,474 ± 20,543 cones/mm2, on average (range 136,001-216,209 cones/mm2). Density dropped anisotropically along the meridians, shallower horizontally, with average best fit parameters (a, b, c) of 61.95, 2.469, 0.268 for horizontal, and 59.11, 2.012, 0.357, for vertical profiles, respectively. In radially averaged profiles, cone density reached 50% of D0 at 151 ± 17 µm eccentricity (range 128-193 µm). Temporal cone density was slightly higher than nasal. Most topographical metrics were highly correlated between fellow eyes.
Conclusions: Despite a 1.6-fold range in absolute cone density, foveolar density profiles could be well described by a sigmoidal decay function across all eyes. This established a normative cone density profile of the healthy foveola. It allowed cone density estimation in cases of only partially available data, which alleviates resolution demands for future studies and renders possible retrospective analyses of foveolar cone topography in sub-optimal imagery.
Conflict of interest statement
Disclosure:
Figures






References
-
- Bringmann A, Syrbe S, Görner K, et al.. The primate fovea: Structure, function and development. Prog Retin Eye Res. 2018; 66: 49–84. - PubMed
-
- Østerberg G. Topography of the Layer of Rods and Cones in the Human Retina: Supplementum. Acta Ophthalmol. 1935.
-
- Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986; 26: 847–855. - PubMed
-
- Tuten WS, Harmening WM. Foveal vision. Curr Biol. 2021; 31(11): R701–R703. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

