Gellan gum formulations containing natural polyphenolic compounds to treat oral candidiasis
- PMID: 40767523
- PMCID: PMC12403768
- DOI: 10.1128/spectrum.00798-25
Gellan gum formulations containing natural polyphenolic compounds to treat oral candidiasis
Abstract
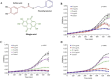
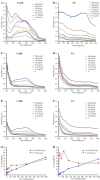
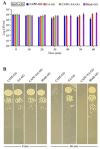
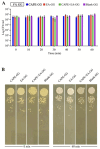
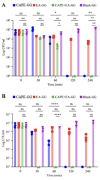
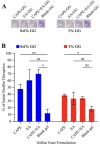
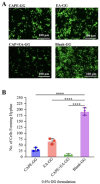
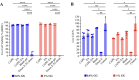
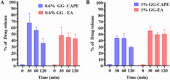
Oral candidiasis (OC) is caused by Candida albicans, targeting immunocompromised individuals and developing drug resistance, highlighting the need for advanced therapeutics. Polyphenols such as caffeic acid phenethyl ester (CAPE) and ellagic acid (EA) display antifungal and immune-modulating properties. Incorporating CAPE and EA in gellan gum (GG) formulation enhances their applicability and effectiveness against OC. We developed GG-based formulations loaded with CAPE (1,000 µg/mL), EA (1,000 µg/mL), or CAPE + EA (1,000 µg/mL each) against C. albicans. GG formulations containing gellan (0.6% and 1.0%), genipin (5 mM), polyethylene glycol 400 (0.5%), and sorbitan monooleate 80 (0.5%) demonstrated enhanced release of CAPE and EA. The 0.6% GG formulation reduced C. albicans CFU by 2-6 log10 within 30 min (P < 0.05) and biofilm mass by 48% (CAPE, P = 0.0034), 60.7% (EA, P = 0.0980), and 70% (CAPE + EA, P = 0.0181). Both 0.6% and 1.0% GG formulations inhibited hyphae (P < 0.0001). GG formulations showed high viability of human red blood cells (92%-94%) and human gingival cells (61%-69%). In artificial chewing simulations (ACS), 0.6% GG exhibited 67.7% (30 min), 55.9% (60 min), and 35.8% (120 min) for CAPE release, and 48.2% (30 min), 45.1% (60 min), and 42.1% (120 min) for EA. In 1% GG, about 44.07% (30 min), 43.8% (60 min), and 29.5% (120 min) of CAPE and 55.8% (30 min), 49.6% (60 min), and 50.6% (120 min) of EA were released. The present study is the first to evaluate the efficacy of CAPE- and EA-loaded GG formulations against C. albicans under ACS, thereby supporting their potential development for OC treatment.IMPORTANCECandida albicans causes OC and presents challenges due to rising antifungal resistance and recurrence in immunocompromised patients. Existing antifungal treatments for OC often fail due to limited bioavailability, short retention times, adverse side effects, and the bitter taste of formulations, impacting patient adherence. CAPE and EA are recognized for their antifungal and immunomodulatory properties but face practical limitations in therapeutic applications, such as poor bioavailability and stability. The present study addresses these challenges by developing GG-based formulations incorporating CAPE and EA. The formulation exhibited significant antifungal efficacy against C. albicans biofilms and hyphal formation, reducing fungal viability under simulated mechanical chewing conditions. These GG-based systems showed minimal cytotoxicity, indicating promising biocompatibility and suitability for oral application. Therefore, the study presents the first report of CAPE/EA-loaded GG formulations under simulated chewing that highlights the importance of innovative therapeutic strategies to improve clinical outcomes in OC treatment.
Keywords: Candida albicans; artificial chewing simulation; biofilm; caffeic acid phenethyl ester; ellagic acid; hyphae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Prevention CfDCa. 2024. Candidiasis. Available from: https://www.cdc.gov/candidiasis. Retrieved Jun 2025.
-
- Erfaninejad M, Zarei Mahmoudabadi A, Maraghi E, Hashemzadeh M, Fatahinia M. 2022. Epidemiology, prevalence, and associated factors of oral candidiasis in HIV patients from southwest Iran in post-highly active antiretroviral therapy era. Front Microbiol 13:983348. doi: 10.3389/fmicb.2022.983348 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

