Patterns of HER2 Expression in Metastatic Prostate and Urothelial Cancers: Implications for HER2-Targeted Therapies
- PMID: 40767528
- PMCID: PMC12375912
- DOI: 10.1158/2767-9764.CRC-25-0069
Patterns of HER2 Expression in Metastatic Prostate and Urothelial Cancers: Implications for HER2-Targeted Therapies
Abstract
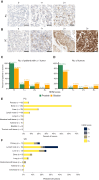
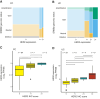
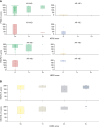
HER2 is an oncogenic driver in multiple cancers and a predictive biomarker for HER2-targeted therapies. Although HER2-directed therapies like fam-trastuzumab deruxtecan are approved for HER2-positive breast and other solid tumors, the landscape of HER2 expression in advanced prostate cancer and urothelial carcinoma remains inadequately characterized. We evaluated HER2 protein expression in metastatic prostate cancer and urothelial carcinoma using a validated IHC assay on tissue microarrays constructed from the University of Washington Tissue Acquisition Necropsy Program. HER2 expression was scored using standardized gastric cancer criteria. Genomic analysis of ERBB2 alterations was conducted on a subset of samples. HER2 expression heterogeneity and its relationship with other surface markers were also evaluated. In the prostate cancer cohort (n = 52 patients, 1-19 tumors per patient), HER2 expression was low, with no 3+ expression observed and only five (10%) patients exhibiting 2+ expression. Low-level ERBB2 copy-number gains were observed in some tumors but did not correlate with HER2 protein expression (P = 0.2). In urothelial carcinoma (n = 20, 2-6 tumors per patient), HER2 expression was more frequent, with ≥2+ expression observed in six (30%) cases and 3+ expression observed in three (15%) cases in at least one tumor. Urothelial carcinoma samples showed less heterogeneity, with more consistent expression across metastases. HER2 overexpression is rare and heterogeneous in metastatic prostate cancer, limiting its utility as a therapeutic target. HER2 expression is more prevalent and uniform in urothelial carcinoma. These findings underscore the importance of comprehensive HER2 assessment in advanced urothelial carcinoma and suggest that success of HER2-directed therapies in prostate cancer will require careful case selection.
Significance: This study demonstrates that HER2 is rarely overexpressed in metastatic prostate cancer but is more common and consistent in urothelial carcinoma. These findings highlight the need for HER2 testing in urothelial cancer and suggest that HER2-targeted therapies in prostate cancer will require careful patient selection.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R. Gulati reports grants from NCI during the conduct of the study. J.L. Wright reports grants from Merck, Nucleix, Seagen, and Janssen, grants and other support from Pacific Edge, and other support from Immunity Bio and UpToDate outside the submitted work. E.Y. Yu reports personal fees from Johnson & Johnson, AstraZeneca, Tolmar, Bristol Myers Squibb, Loxo, Novartis, and Aadi Bioscience, grants and personal fees from Lantheus, Merck, and Bayer, and grants from Dendreon, Pfizer, Blue Earth, and Tyra outside the submitted work. J.E. Hawley reports other from Johnson & Johnson outside the submitted work. H.H. Cheng reports grants from Clovis Oncology, Janssen, Promontory Therapeutics, Medivation, and Sanofi outside the submitted work. M.T. Schweizer reports personal fees from Sanofi, Fibrogen, and Daiichi Sankyo, grants from Novartis, Zenith Epigenetics, Eli Lilly and Company, Bristol Myers Squibb, Merck, Immunomedics, Tmunity, SingalOne Bio, Epigenetix, Xencor, Incyte, Ambrx, AstraZeneca, and Oric Pharmaceuticals, and grants and personal fees from Janssen and Pfizer outside the submitted work. P. Grivas reports grants and personal fees from Bristol Myers Squibb, MSD, EMD Serono, and G1 Therapeutics, grants, personal fees, and nonfinancial support from Gilead Sciences, grants from Acrivon Therapeutics, ALX Oncology, Genentech, Mirati Therapeutics, and QED Therapeutics, and personal fees from AstraZeneca, Pfizer, Janssen, Roche, Astellas Pharma, Fresenius Kabi, PureTech, Aadi Bioscience, CG Oncology, Strata Oncology, ImmunityBio, Asieris Pharmaceuticals, AbbVie, Bicycle Therapeutics, Replimune, Daiichi Sankyo, Foundation Medicine, Urogen, Eli Lilly and Company, and Tyra Biosciences outside the submitted work. P.S. Nelson reports personal fees from Janssen, Genentech, AstraZeneca, and Pfizer and grants from Janssen outside the submitted work. M.C. Haffner reports other support from Novartis, Genentech, Bristol Myers Squibb, and Promicell and personal fees from Pfizer and AstraZeneca outside the submitted work. R. Raychaudhuri reports grants from NIH/NCI during the conduct of the study. No disclosures were reported by the other authors.
Figures
Similar articles
-
HER2 overexpression in urothelial carcinoma with GATA3 and PPARG copy number gains.Oncologist. 2024 Aug 5;29(8):e1094-e1097. doi: 10.1093/oncolo/oyae127. Oncologist. 2024. PMID: 38908022 Free PMC article.
-
Risk factors and therapeutic significance of HER2 overexpression in urothelial carcinoma.Pathol Res Pract. 2025 Aug;272:156079. doi: 10.1016/j.prp.2025.156079. Epub 2025 Jun 9. Pathol Res Pract. 2025. PMID: 40513427
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
HER2DX and survival outcomes in early-stage HER2-positive breast cancer: an individual patient-level meta-analysis.Lancet Oncol. 2025 Aug;26(8):1100-1112. doi: 10.1016/S1470-2045(25)00276-1. Epub 2025 Jul 14. Lancet Oncol. 2025. PMID: 40675165
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 1998;20:41–8. - PubMed
-
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505–16. - PubMed
-
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017;389:2415–29. - PubMed
MeSH terms
Substances
Grants and funding
- Institute for Prostate Cancer Research (IPCR)
- 2021184/Doris Duke Charitable Foundation (DDCF)
- R21 CA277368/CA/NCI NIH HHS/United States
- W81XWH-20-1-0111/U.S. Department of Defense (DOD)
- P30 CA015704/CA/NCI NIH HHS/United States
- S10 OD028685/OD/NIH HHS/United States
- R01 CA234715/CA/NCI NIH HHS/United States
- V Foundation for Cancer Research (VFCR)
- R37CA286450/National Cancer Institute (NCI)
- W81XWH-21-1-0264/U.S. Department of Defense (DOD)
- W81XWH-21-1-0229/U.S. Department of Defense (DOD)
- PC-SYNERGY/Prostate Cancer Foundation (PCF)
- R37 CA286450/CA/NCI NIH HHS/United States
- R01 CA266452/CA/NCI NIH HHS/United States
- P50CA097186/National Cancer Institute (NCI)
- R50 CA221836/CA/NCI NIH HHS/United States
- P50 CA097186/CA/NCI NIH HHS/United States
- R01 CA280056/CA/NCI NIH HHS/United States
- R50CA274336/National Cancer Institute (NCI)
- R50 CA274336/CA/NCI NIH HHS/United States
- R50CA221836/National Cancer Institute (NCI)
- R21CA277368/National Cancer Institute (NCI)
- R01CA276308/National Cancer Institute (NCI)
- R35CA274442/National Cancer Institute (NCI)
- HT94252410755/U.S. Department of Defense (DOD)
- R01CA266452/National Cancer Institute (NCI)
- R50CA22183/National Cancer Institute (NCI)
- RS-2024-00334566/National Research Foundation of Korea (NRF)
- K08 CA282978/CA/NCI NIH HHS/United States
- R35 CA274442/CA/NCI NIH HHS/United States
- R37 CA230617/CA/NCI NIH HHS/United States
- W81XWH-22-1-0278/U.S. Department of Defense (DOD)
- W81XWH-17-2-0043/U.S. Department of Defense (DOD)
- R37CA230617/National Cancer Institute (NCI)
- P01 CA163227/CA/NCI NIH HHS/United States
- W81XWH-18-1-0689/U.S. Department of Defense (DOD)
- R01 CA276308/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

