Serum- and glucocorticoid-induced kinase 3 orchestrates glucocorticoid signaling to facilitate chromatin remodeling during murine adipogenesis
- PMID: 40767604
- PMCID: PMC12483569
- DOI: 10.1172/JCI186534
Serum- and glucocorticoid-induced kinase 3 orchestrates glucocorticoid signaling to facilitate chromatin remodeling during murine adipogenesis
Abstract
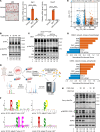
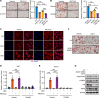
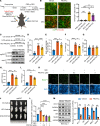
Elevated glucocorticoid levels are common in conditions such as aging, chronic stress, Cushing syndrome, and glucocorticoid therapy. While glucocorticoids suppress inflammation through the glucocorticoid receptor (GR), they also cause metabolic side effects. Investigating alternative pathways beyond GR activation is crucial for reducing these side effects. Our phosphoproteomics analysis revealed that glucocorticoid exposure promotes phosphorylation at the RxxS motifs of multiple proteins in preadipocytes, including those mediated by serum- and glucocorticoid-induced kinase 3 (SGK3). SGK3 is a key mediator of glucocorticoid-induced adipogenesis, as shown by impaired adipogenesis after SGK3 inhibition or genetic ablation. Sgk3-KO mice were resistant to obesity induced by glucocorticoid or a high-fat diet, and proteolysis targeting chimeras (PROTAC) targeting SGK3 reduced adipogenesis in both obese mice and in a thyroid eye disease cell line. Mechanistically, SGK3 translocated to the nucleus upon glucocorticoid stimulation, interacted with and phosphorylated the BRG1 subunit of the BAF complex, and prevented BRG1 degradation, promoting chromatin remodeling necessary for adipogenesis. These findings highlight SGK3 as a potential therapeutic target to mitigate metabolic side effects of elevated glucocorticoid levels.
Keywords: Adipose tissue; Cell biology; Metabolism; Obesity.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

