Bridging verbal coordination and neural dynamics
- PMID: 40768245
- PMCID: PMC12327942
- DOI: 10.7554/eLife.99547
Bridging verbal coordination and neural dynamics
Abstract
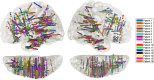
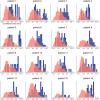
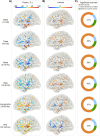
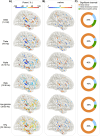
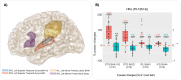
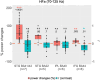
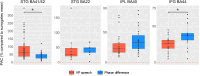
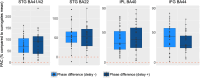
Our use of language, which is profoundly social in nature, essentially takes place in interactive contexts and is shaped by precise coordination dynamics that interlocutors must observe. Thus, language interaction is highly demanding on fast adjustment of speech production. Here, we developed a real-time coupled-oscillators virtual partner (VP) that allows - by changing the coupling strength parameters - to modulate the ability to synchronise speech with a virtual speaker. Then, we recorded the intracranial brain activity of 16 patients with drug-resistant epilepsy while they performed a verbal coordination task with the VP. More precisely, patients had to repeat short sentences synchronously with the VP. This synchronous speech task is efficient to highlight both the dorsal and ventral language pathways. Importantly, combining time-resolved verbal coordination and neural activity shows more spatially differentiated patterns and different types of neural sensitivity along the dorsal pathway. More precisely, high-frequency activity (HFa) in left secondary auditory regions is highly sensitive to verbal coordinative dynamics, while primary regions are not. Finally, while bilateral engagement was observed in the HFa of the inferior frontal gyrus BA44 - which seems to index online coordinative adjustments that are continuously required to compensate deviation from synchronisation - interpretation of right hemisphere involvement should be approached cautiously due to relatively sparse electrode coverage. These findings illustrate the possibility and value of using a fully dynamic, adaptive, and interactive language task to gather deeper understanding of the subtending neural dynamics involved in speech perception, production as well as their interaction.
Keywords: human; inferior frontal gyrus; mutual adaptation; neurals dynamics; neuroscience; speech coordination; superior temporal gyrus; synchronous speech.
© 2024, Schwab-Mohamed et al.
Conflict of interest statement
IS, MM, AT, BM, LL, DS No competing interests declared
Figures


Update of
- doi: 10.1101/2024.04.23.590817
- doi: 10.7554/eLife.99547.1
- doi: 10.7554/eLife.99547.2
- doi: 10.7554/eLife.99547.3
References
-
- Bögels S, Levinson SC. The brain behind the response: insights into turn-taking in conversation from neuroimaging. Research on Language and Social Interaction. 2017;50:71–89. doi: 10.1080/08351813.2017.1262118. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

