Impaired Secondary Platelet Response in Chronic Kidney Disease as a Consequence of Prior Platelet Activation
- PMID: 40768837
- PMCID: PMC12346058
- DOI: 10.1016/j.jacbts.2025.101355
Impaired Secondary Platelet Response in Chronic Kidney Disease as a Consequence of Prior Platelet Activation
Abstract
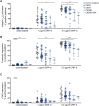
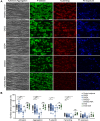
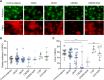
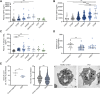
Patients with chronic kidney disease (CKD) are at increased risk of thrombotic and hemorrhagic complications. Findings on platelet defects in CKD are conflicting. Therefore, we examined platelet function in CKD stage 3 to 5 dialysis patients without antithrombotic therapy, in CKD5D/hemodialysis patients on acetylsalicylic acid (ASA) as well as in a CKD mouse model. Patients with advanced CKD without antithrombotic therapy show platelet preactivation with a partial secondary platelet dysfunction mainly upon collagen/GPVI stimulation. Platelets from hemodialysis patients on ASA showed a less severe CKD-associated secondary platelet dysfunction compared with those not taking ASA, with comparable observations in CKD mice on ASA vs vehicle.
Keywords: acetylsalicylic acid; bleeding; chronic kidney disease; platelets; thrombosis.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was financially supported by the Alexander von Humboldt Foundation (to Dr Baaten); the Dutch Heart Foundation (2020T020, to Dr Baaten); the START-Program of the Faculty of Medicine of the RWTH Aachen University (105/20 to Drs Baaten and Noels), the German Heart Foundation/German Foundation of Heart Research (F/61/23, Dr Baaten), the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University (PTD 1-11 to Dr Kahles and 1-12 to Dr Noels) and by the German Research Foundation (DFG) Project-ID 322900939 SFB/TRR219 (S-03, C-01, C-04, C-05, M-05 and M-07 to Drs, Noels, Kramann, Marx, Floege, Kahles, Speer, and Schunk), Project-ID 403224013 – SFB 1382 (A-04 to Drs Jankowski and Noels) and Project-ID 520275106 Emmy Noether Research Group (Dr Kahles). Dr Henning received a Kaltenbach Stipendium from the Deutsche Herzstiftung. Further funding was provided by the “Else Kröner-Fresenius-Stiftung” (Project 2020_EKEA.60 to Dr Noels, and 2022_EKES.03 to Dr Saritas) and the German Centre for Cardiovascular Research (DZHK-B23Ex to Dr Noels). Dr Boor was supported by the German Research Foundation (DFG, Project IDs 322900939, 454024652, 432698239, and 445703531), European Research Council (ERC Consolidator Grant No 101001791), and the Federal Ministry of Education and Research (BMBF, STOP-FSGS-01GM2202C). Dr Kahles was additionally funded by the European Research Area Network on Cardiovascular Diseases (ERA-CVD and BMBF, Grant No. JTC-2019, MyPenPath - 01KL2004) and the European Foundation for the Study of Diabetes (EFSD)/Novo Nordisk Foundation (NNF2OSA0066111). Dr Jankowski also reports funding from COST Action PerMedik, CA21165, supported by COST (European Cooperation in Science and Technology). Dr Kurt has served as a speaker for SphingoTec/14-4 Pharmaceuticals; and received travel support from 14-4 Pharmaceuticals and Novo Nordisk. Drs Jankowski, Marx, and Noels are founding shareholders of AMICARE Development GmbH. Dr Kahles has served as a speaker for Novo Nordisk, Lilly, AstraZeneca, and DGK-Akademie; consulted for Novo Nordisk, Bayer, and PricewaterhouseCoopers/Strategy; and received travel support from Amgen, Novo Nordisk, Boehringer Ingelheim, Bayer, SphingoTec/14-4, and Lilly. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. - PubMed
-
- Boccardo P., Remuzzi G., Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. - PubMed
-
- Ocak G., Rookmaaker M.B., Algra A., et al. Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: a cohort study. J Thromb Haemost. 2018;16:65–73. - PubMed
LinkOut - more resources
Full Text Sources

