Group optimization methods for dose planning in tES
- PMID: 40769162
- PMCID: PMC12351633
- DOI: 10.1088/1741-2552/adf887
Group optimization methods for dose planning in tES
Abstract
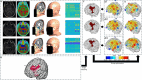
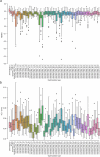
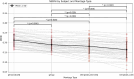
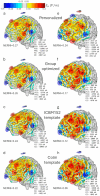
Objective.Optimizing transcranial electrical stimulation (tES) parameters-including stimulator settings and electrode placements, using magnetic resonance imaging-derived head models is essential for achieving precise electric field (E-field) distributions, enhancing therapeutic efficacy, and reducing inter-individual variability. However, the dependence on individually personalized MRI-based models limits their scalability in some clinical and research contexts. To overcome this limitation, we propose a novel group-level optimization framework employing multiple representative head models.Approach.The proposed optimization approach utilizes computational modeling based on multiple representative head models, selected to minimize group-level error compared to baseline (no stimulation). This method effectively balances focal stimulation intensity within targeted brain regions while minimizing off-target effects. We evaluated our method through computational modeling and leave-one-out cross-validation using data from 54 subjects, and analyzed the effectiveness, generalizability, and predictive utility of anatomical characteristics.Main results.Group-optimized protocols significantly outperformed standard template-based approaches when within-subject variability was accounted for using paired analyzes. Although average performance differences appeared modest in aggregate comparisons, paired statistical tests revealed that group-based solutions yielded systematically better targeting across participants. Additionally, group protocols consistently reduced the occurrence of poor outcomes observed with some templates. Correlations between anatomical features (e.g. head perimeter and tissue volumes) and E-field parameters revealed predictive relationships. This insight enables further optimization improvements through the strategic selection of representative head models that are electro-anatomically similar to the target subjects. Importantly, this approach eliminates the need fora prioriselection of a single representative template, offering a scalable and more flexible alternative when individualized MRI-based models are not available.Significance.The proposed group optimization framework provides a scalable and robust alternative to personalized approaches, substantially enhancing the feasibility and accessibility of model-driven tES protocols in diverse clinical and research environments.
Keywords: computational models; dose parameter; transcranial electrical stimulation.
Creative Commons Attribution license.
Conflict of interest statement
Dr Ricardo Salvador works for Neuroelectrics Barcelona SLU, a company developing computationally-driven brain stimulation solutions. Dr Giulio Ruffini works for and is a co-founder of Neuroelectrics and holds several patents in model-driven non-invasive brain stimulation.
Figures

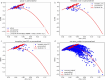
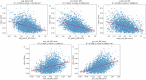
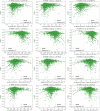
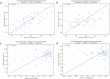
Update of
-
Group optimization methods for dose planning in tES.bioRxiv [Preprint]. 2025 Mar 19:2025.03.18.643934. doi: 10.1101/2025.03.18.643934. bioRxiv. 2025. Update in: J Neural Eng. 2025 Aug 14;22(4). doi: 10.1088/1741-2552/adf887. PMID: 40166293 Free PMC article. Updated. Preprint.
References
-
- Aydin Ü, Rampp S, Wollbrink A, Kugel H, Cho J, Knosche T R, Grova C, Wellmer J, Wolters C H. Zoomed MRI guided by combined EEG/MEG source analysis: a multimodal approach for optimizing presurgical epilepsy work-up and its application in a multi-focal epilepsy patient case study. Brain Topogr. 2017;30:417–33. doi: 10.1007/s10548-017-0568-9. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
