Oxytocin Receptor Expression and Activation in Parasympathetic Brainstem Cardiac Vagal Neurons
- PMID: 40769582
- PMCID: PMC12376954
- DOI: 10.1523/ENEURO.0204-25.2025
Oxytocin Receptor Expression and Activation in Parasympathetic Brainstem Cardiac Vagal Neurons
Abstract
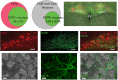
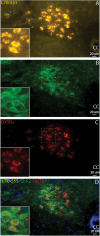
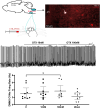
Autonomic imbalance-particularly reduced activity from brainstem parasympathetic cardiac vagal neurons (CVNs)-is a major characteristic of many cardiorespiratory diseases. Therapeutic approaches to selectively enhance CVN activity have been limited by the lack of defined, translationally relevant targets. Previous studies have identified an important excitatory synaptic pathway from oxytocin (OXT) neurons in the paraventricular nucleus of the hypothalamus to brainstem CVNs, suggesting that OXT could provide a key selective excitation of CVNs. In clinical studies, intranasal OXT has been shown to increase parasympathetic cardiac activity, improve autonomic balance, and reduce obstructive event durations and oxygen desaturations in obstructive sleep apnea patients. However, the mechanisms by which activation of hypothalamic OXT neurons, or intranasal OXT, enhance brainstem parasympathetic cardiac activity remain unclear. CVNs are located in two cholinergic brainstem nuclei: nucleus ambiguus (NA) and dorsal motor nucleus of the vagus (DMNX). In this study, we characterize the colocalization of OXT receptors (OXTRs) in both CVNs and non-CVN cholinergic neurons in the male and female mouse NA and DMNX nuclei. We found that OXT receptors are highly expressed in CVNs in the DMNX, but not in the NA. OXT increases the firing of DMNX CVN, with no effect on NA CVNs. Selective chemogenetic excitation of OXTR+ CVNs in the DMNX-achieved by a combination of Cre- and flp-dependent DREADD expression-evoked a rapid and sustained bradycardia. These findings suggest that activation of DMNX CVNs expressing OXTR with oxytocin may represent a novel translational therapeutic target for restoring autonomic balance in cardiorespiratory disorders.
Keywords: DMNX and NA; DREADDs; autonomic regulation; cardiac vagal neurons; chemogenetic; oxytocin receptors.
Copyright © 2025 Wang et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Update of
-
Oxytocin Receptor Expression and Activation in Parasympathetic Brainstem Cardiac Vagal Neurons.bioRxiv [Preprint]. 2025 Mar 20:2025.03.19.644204. doi: 10.1101/2025.03.19.644204. bioRxiv. 2025. Update in: eNeuro. 2025 Aug 22;12(8):ENEURO.0204-25.2025. doi: 10.1523/ENEURO.0204-25.2025. PMID: 40166340 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
