Plasmodium falciparum egress disrupts endothelial junctions and activates JAK-STAT signaling in a microvascular 3D blood-brain barrier model
- PMID: 40769972
- PMCID: PMC12328663
- DOI: 10.1038/s41467-025-62514-2
Plasmodium falciparum egress disrupts endothelial junctions and activates JAK-STAT signaling in a microvascular 3D blood-brain barrier model
Abstract
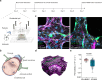
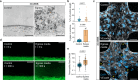
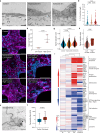
Cerebral malaria is a severe neurovascular complication of Plasmodium falciparum infection, with high mortality rates even after treatment with effective antimalarials. Limitations in current experimental models have hindered our knowledge of the disease. We developed a 3D blood-brain barrier (BBB) model with enhanced barrier properties using primary brain endothelial cells, astrocytes, and pericytes. Exposure to parasite egress products increases microvascular permeability, likely due to transcriptional downregulation of junctional and vascular development genes in endothelial cells. In addition, it increases the expression of ferroptosis markers, antigen presentation and type I interferon genes and upregulates the JAK-STAT pathway across all BBB cell types. Incubation with cytoadherent schizont-stage P. falciparum-infected erythrocytes induces a similar, but highly localized transcriptional shift, along with inter-endothelial gaps at sites of parasite egress, leading to enhanced permeability. Treatment with the JAK-STAT inhibitor Ruxolitinib prevents the increase in permeability induced by P. falciparum egress products. These findings provide key insights into the parasite-mediated mechanisms driving brain microvascular pathogenesis in cerebral malaria and suggest potential avenues for adjunctive therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- World malaria report 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
-
- Taylor, T. E. et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med.10, 143–145 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

