Novel CDK2/CDK9 inhibitor fadraciclib targets cell survival and DNA damage pathways and synergizes with encorafenib in human colorectal cancer cells with BRAF(V600E)
- PMID: 40769976
- PMCID: PMC12329046
- DOI: 10.1038/s41389-025-00574-1
Novel CDK2/CDK9 inhibitor fadraciclib targets cell survival and DNA damage pathways and synergizes with encorafenib in human colorectal cancer cells with BRAF(V600E)
Abstract
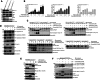
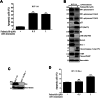
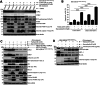
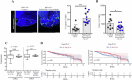
The oncogenic BRAF(V600E) mutation activates the ERK1/2 pathway and is detected in 10% of human colorectal cancers (CRCs) where it is associated with poor prognosis. Inhibitors of BRAF have shown only modest efficacy in patients with CRC due to intrinsic drug resistance. We studied the CDK2/CDK9 inhibitor, fadraciclib, alone and in combination with the BRAF inhibitor encorafenib in isogenic human RKO CRC cells with two, one, or no BRAFV600E alleles (RKO+/+, A19+/-, T29-/-) and in BRAF wild-type HCT-116 cells, including Bax knockout HCT-116Bax-/- cells. Treatment with fadraciclib was shown to suppress MCL-1 and phospho-MCL-1 (Ser64), induce a Bax-dependent apoptosis, and inhibit colony formation in a BRAF gene dose-dependent manner. Fadraciclib decreased phosphorylation of RNA polymerase II, indicating suppression of RNA transcription. The tumor growth inhibitory effect of fadraciclib plus encorafenib was synergistic. Fadraciclib decreased Rb phosphorylation, inhibited cell cycle progression, and promoted DNA damage as evidenced by cleavage of PARP, increased pH2AX (ser139), and activation of p53. In RKO+/+ versus A19+/- or T29-/- cells, drug treatment was associated with greater suppression of p-Rb and inhibition of apoptosis and the cell cycle. In a zebrafish xenograft model, fadraciclib plus encorafenib significantly reduced tumor size, concurrent with increased caspase-3 activation. In human CRCs, BRAF mutation was associated with overexpression of CDK2, and CDK9 overexpression was associated with worse patient survival. In conclusion, fadraciclib depletes MCL-1 to potentiate apoptosis and, combined with encorafenib, synergistically suppresses tumor cell growth in a BRAFV600E gene dose-dependent manner. These data suggest a novel therapeutic strategy in CRCs with BRAFV600E.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. In vivo experiments were performed using zebrafish (Danio rerio), which were handled according to European animal welfare Legislation, Directive 2010/63/EU (European Commission, 2016), DGAV-ORBEA 004166/0421/000/000/2020. No human research was conducted in this manuscript.
Figures


References
-
- Tabernero J, Ros J, Elez E. The evolving treatment landscape in BRAF-V600E-mutated metastatic colorectal cancer. Am Soc Clin Oncol Educ Book. 2022;42:1–10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

