Immunosenescence: signaling pathways, diseases and therapeutic targets
- PMID: 40769978
- PMCID: PMC12328749
- DOI: 10.1038/s41392-025-02371-z
Immunosenescence: signaling pathways, diseases and therapeutic targets
Abstract
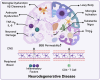
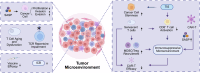
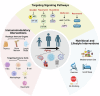
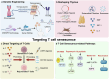
Immunosenescence refers to the abnormal activation or dysfunction of the immune system as people age. Inflammaging is a typical pathological inflammatory state associated with immunosenescence and is characterized by excessive expression of proinflammatory cytokines in aged immune cells. Chronic inflammation contributes to a variety of age-related diseases, such as neurodegenerative disease, cancer, infectious disease, and autoimmune diseases. Although not fully understood, recent studies contribute greatly to uncovering the underlying mechanisms of immunosenescence at the molecular and cellular levels. Immunosenescence is associated with dysregulated signaling pathways (e.g., overactivation of the NF-κB signaling pathway and downregulation of the melatonin signaling pathway) and abnormal immune cell responses with functional alterations and phenotypic shifts. These advances remarkably promote the development of countermeasures against immunosenescence for the treatment of age-related diseases. Some anti-immunosenescence treatments have already shown promising results in clinical trials. In this review, we discuss the molecular and cellular mechanisms of immunosenescence and summarize the critical role of immunosenescence in the pathogenesis of age-related diseases. Potential interventions to mitigate immunosenescence, including reshaping immune organs, targeting different immune cells or signaling pathways, and nutritional and lifestyle interventions, are summarized. Some treatment strategies have already launched into clinical trials. This study aims to provide a systematic and comprehensive introduction to the basic and clinical research progress of immunosenescence, thus accelerating research on immunosenescence in related diseases and promoting the development of targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Consent for publication: All the authors have read and approved the final manuscript.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

