Neutrophil-to-Lymphocyte ratio as surrogate for JAK2V617F suppression and event-free survival in polycythemia vera
- PMID: 40770048
- PMCID: PMC12328675
- DOI: 10.1038/s41408-025-01317-6
Neutrophil-to-Lymphocyte ratio as surrogate for JAK2V617F suppression and event-free survival in polycythemia vera
Abstract
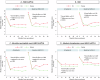
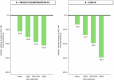
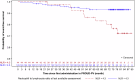
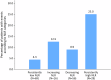
Chronic systemic inflammation is a key driver of polycythemia vera (PV) progression, but the immunomodulatory effects of current treatments remain poorly defined. The neutrophil-to-lymphocyte ratio (NLR) is an accessible biomarker of systemic inflammation proven in other contexts, but its role in monitoring PV disease activity has not been established. Using data from three of the largest PV clinical trials, we evaluated the effects of PV therapies on NLR and its relationship with molecular response and clinical outcomes. In 404 hematocrit-controlled patients from the ECLAP study, hydroxyurea (HU) failed to significantly lower NLR (p = 0.11) due to the parallel declines in ANC and ALC. Neither leukocyte counts nor NLR were significantly reduced by phlebotomy in ECLAP patients treated without cytoreductive therapy. In contrast, the Low-PV study showed that while phlebotomy tended to increase NLR, low-dose ropeginterferon alfa-2b (Ropeg) significantly reduced NLR (-18.2% and -36.3% in patients with low and high baseline NLR, respectively) by suppressing ANC rather than lymphocytes. NLR reduction correlated with the primary Low-PV endpoint (p = 0.021) and reduction of JAK2 variant allele frequency (VAF) [1]. The PROUD-PV/CONTINUATION-PV study confirmed the superior effect of Ropeg over HU, with a significantly greater NLR reduction at 60 months (-56.5% versus -33.6%, respectively, p = 0.019) in patients with high baseline NLR. Moreover, NLR reduction was associated with decreased JAK2V617F VAF (p < 0.0001) and improved event-free survival (p = 0.010). These findings identify NLR as a dynamic biomarker of treatment response and prognosis in PV and support its incorporation into routine monitoring.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: TB: Research grant from GSK and AOP. Advisory Board AOP and Italfarmaco. VE and CK are employees of AOP Orphan Pharmaceuticals. GGL: Speaker’s bureau from Novartis and GSK. AI: Speaker honoraria from AOP Health, BMS, GSK, Incyte, Novartis and Pfizer. ER: Advisory Board AOP Health. VDS: Advisory Board AOP Health. PG: Advisory Board Novartis, Incyte and GSK; Speaker’s bureau for Novartis, Gsk, Abbvie, AOP. AMV: Advisory Board and/or lectures from Novartis, AbbVie, AOP Pharmaceuticals, BMS and Incyte. HG: grants and/or personal fees from AOP Health, Novartis, and BMS-Pharma. AR: fees for consultancies and participation in meetings, boards, and symposia sponsored by Amgen, Pfizer, Novartis, Kite‐Gilead, Jazz, Astellas, Abbvie, Incyte, and Omeros. JMS: Advisory Board or Consultant for Abbvie, Incyte, Protagonist, Novartis, SDP Oncology and Karyopharm. AG, FF, AC and DC have no conflicts of interest to declare.
Figures



References
-
- Barbui T, Carobbio A, Guglielmelli P, Ghirardi A, Fenili F, Loscocco GG, et al. Neutrophil/lymphocyte ratio identifies low-risk polycythaemia vera patients for early Ropeginterferon alfa-2b therapy. Br J Haematol. 2024;205:2287–94. - PubMed
-
- Tefferi A, Barbui T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:1465–87. - PubMed
-
- Gerds AT, Gotlib J, Ali H, Bose P, Dunbar A, Elshoury A, et al. Myeloproliferative Neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1033–62. - PubMed
-
- Marchetti M, Vannucchi AM, Griesshammer M, Harrison C, Koschmieder S, Gisslinger H, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022;9:e301–e311. - PubMed
-
- Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

