Lithium deficiency and the onset of Alzheimer's disease
- PMID: 40770094
- PMCID: PMC12443616
- DOI: 10.1038/s41586-025-09335-x
Lithium deficiency and the onset of Alzheimer's disease
Abstract
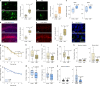
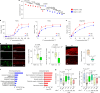
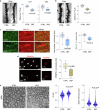
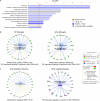
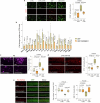
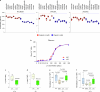
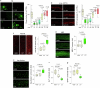
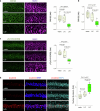
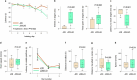
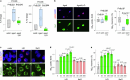
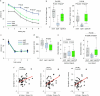
The earliest molecular changes in Alzheimer's disease (AD) are poorly understood1-5. Here we show that endogenous lithium (Li) is dynamically regulated in the brain and contributes to cognitive preservation during ageing. Of the metals we analysed, Li was the only one that was significantly reduced in the brain in individuals with mild cognitive impairment (MCI), a precursor to AD. Li bioavailability was further reduced in AD by amyloid sequestration. We explored the role of endogenous Li in the brain by depleting it from the diet of wild-type and AD mouse models. Reducing endogenous cortical Li by approximately 50% markedly increased the deposition of amyloid-β and the accumulation of phospho-tau, and led to pro-inflammatory microglial activation, the loss of synapses, axons and myelin, and accelerated cognitive decline. These effects were mediated, at least in part, through activation of the kinase GSK3β. Single-nucleus RNA-seq showed that Li deficiency gives rise to transcriptome changes in multiple brain cell types that overlap with transcriptome changes in AD. Replacement therapy with lithium orotate, which is a Li salt with reduced amyloid binding, prevents pathological changes and memory loss in AD mouse models and ageing wild-type mice. These findings reveal physiological effects of endogenous Li in the brain and indicate that disruption of Li homeostasis may be an early event in the pathogenesis of AD. Li replacement with amyloid-evading salts is a potential approach to the prevention and treatment of AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- James, B. D. & Bennett, D. A. Causes and patterns of dementia: an update in the era of redefining Alzheimer’s disease. Annu. Rev. Public Health40, 65–84 (2019). - PubMed
-
- De Strooper, B. & Karran, E. The cellular phase of Alzheimer’s disease. Cell164, 603–615 (2016). - PubMed
-
- Mathys, H. et al. Single-cell multiregion dissection of Alzheimer’s disease. Nature10.1038/s41586-024-07606-7 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

