Real-world clinical outcomes associated with first-line palbociclib and aromatase inhibitor therapy among patients with HR+/HER2- advanced breast cancer in Europe
- PMID: 40770162
- PMCID: PMC12396976
- DOI: 10.1007/s10549-025-07707-5
Real-world clinical outcomes associated with first-line palbociclib and aromatase inhibitor therapy among patients with HR+/HER2- advanced breast cancer in Europe
Abstract
Purpose: Cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) combined with endocrine therapy is the recommended first-line (1L) treatment for hormone receptor-positive and human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer (ABC). Real-world evidence (RWE) describing 1L CDK4/6i regimens and associated clinical outcomes in Europe is limited. The study objective was to describe clinical characteristics, tumor response, and survival outcomes in patients with HR+/HER2- ABC.
Methods: This retrospective, observational cohort study used data from 52 treatment centers in the UK, Spain, and Germany and included patients who initiated 1L palbociclib + aromatase inhibitor (AI) therapy for ABC between 2016 and 2020. Primary endpoints were real-world progression-free survival (rwPFS) and overall survival (OS).
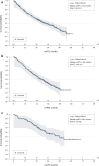
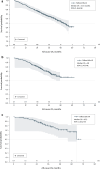
Results: Data were abstracted from 856 patients. During treatment, complete response, partial response, or stable disease was achieved for 86.1% of patients in Spain, 80.7% in the UK, and 79.0% in Germany, while complete or partial response was achieved for 43.8% of patients in Spain, 34.9% in the UK, and 16.9% in Germany. Median rwPFS during treatment was 28.1 months for patients in Spain, 33.9 months in the UK, and 48.1 months in Germany. Median OS was 51.3 months (95% CI 46.6-NE) in the UK, 65.2 months (95% CI 65.2-NE) in Germany, and not reached in Spain.
Conclusion: This RWE supports the clinical effectiveness of 1L palbociclib + AI in routine clinical practice in European countries-consistent with the efficacy observed in clinical trials-and further supports the implementation of palbociclib-based regimens as frontline treatment of HR+/HER2- ABC.
Keywords: CDK4/6 inhibitor; Europe; HR+/HER2− advanced breast cancer; Palbociclib; Real-world evidence; Real-world progression-free survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: RCP, MIJ, KLD, AH, VDA, and GF are employees of RTI Health Solutions, an independent nonprofit research organization which was a paid consultant to Pfizer in connection with conduct of the study and development of this manuscript. EIB, JD, CC, and BL are employees and shareholders of Pfizer, Inc., the sponsor of this study. OO is a member of advisory boards for and/or received honoraria from Pfizer, AstraZeneca, Novartis, Exact Sciences, Eisai, Daiichi Sankyo, Roche, Gilead, Eli Lilly, Merck, and Tesaro; received travel grants from Pfizer, AstraZeneca, Novartis, Eisai, Roche, Amgen, Gilead, Eli Lilly, and Menarini; and received research funding from Pfizer, Novartis, Roche, MedDiagnostics, Make2 Count, BCI, and Genomic Health. MJB has no conflicts to report. EG has received consulting fees from Roche, Pfizer, Eli Lilly, Daicchi-Sankyo, Gilead, and AstraZeneca. AW is a member of advisory boards for Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer, Roche, Tesaro, Sirtex, MSD, Pierre Fabre, Clovis Oncology, Organon, Seagen, Exact Sciences, Gilead, and Daiichi Sankyo. Ethics approval and informed consent: The RTI International Institutional Review Board deemed this study exempt from full review. The study was approved by country-specific ethics review boards in Germany, Spain, and the UK. Due to the use of deidentified/pseudonymized data, an informed consent waiver was approved.
Figures
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263 - PubMed
-
- European Cancer Information System (2022) Breast Cancer in the EU. https://ecis.jrc.ec.europa.eu/pdf/Breast_cancer_2022-Oct_2023.pdf. Accessed 13 Nov 2023
-
- Office for National Statistics (2019) Cancer survival in England-adults diagnosed. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/.... Accessed 13 Nov 2023
-
- American Cancer Society (2022) Breast cancer facts and figures 2022–2024. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-.... Accessed 13 Nov 2023
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

