Temporal characteristics of hemodynamic responses during active and passive hand movements in schizophrenia spectrum disorder
- PMID: 40770189
- PMCID: PMC12328563
- DOI: 10.1038/s41537-025-00654-6
Temporal characteristics of hemodynamic responses during active and passive hand movements in schizophrenia spectrum disorder
Abstract
In healthy individuals, active hand-movements typically elicit earlier neural processing than passive one, reflected by more positive contrast estimates of the first-order temporal derivative (TD) of hemodynamic response function (HRF) in functional MRI (fMRI) analyses. This temporal advantage might be due to prior movement-awareness and predictive mechanisms that support self-other distinction. However, it is unknown whether impaired predictive mechanisms in Schizophrenia Spectrum Disorder (SSD) influence earlier neural processing. Patients with SSD (n = 20) and healthy controls (HC; n = 20) performed active and passive hand movements, while detected delays in video feedback of their own or another person's hand. The recorded fMRI data were analysed applying TD to examine timing and second-order dispersion derivative (DD) to evaluate duration of neural responses. Compared to HC, patients with SSD exhibited delayed BOLD responses during active vs. passive movements in the right caudate nucleus, lobule VIII of right cerebellar hemisphere, left superior temporal gyrus, left postcentral gyrus, left thalamus, and left putamen/insula. Furthermore, during active movement with own hand feedback, patients with SSD showed delayed activation in the bilateral putamen and insula. Delayed insula/putamen responses' were associated with symptom severity. However, these exploratory findings remain not significant after correction for multiple comparisons and attenuated with Spearman's-rank correlations. Delayed BOLD responses in patients with SSD, particularly in the right cerebellar lobule VIII, left thalamus, and bilateral insula/putamen may contribute to disturbances in the sense of agency. Altered timing/duration of neural responses reflects new insight underlying deficits in predictive and feedback-monitoring mechanisms in SSD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


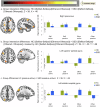
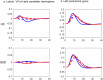
References
-
- Voss, M. et al. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain133, 3104–3112 (2010). - PubMed
Grants and funding
- 286893149/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 222641018/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 286893149/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 222641018/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 286893149/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources

