Hypoxia ameliorates neurodegeneration and movement disorder in a mouse model of Parkinson's disease
- PMID: 40770507
- PMCID: PMC12411263
- DOI: 10.1038/s41593-025-02010-4
Hypoxia ameliorates neurodegeneration and movement disorder in a mouse model of Parkinson's disease
Abstract
Parkinson's disease (PD) is characterized by inclusions of α-synuclein (α-syn) and mitochondrial dysfunction in dopaminergic (DA) neurons of the substantia nigra pars compacta (SNpc). Patients with PD anecdotally experience symptom improvement at high altitude; chronic hypoxia prevents the development of Leigh-like brain disease in mice with mitochondrial complex I deficiency. Here we report that intrastriatal injection of α-syn preformed fibrils (PFFs) in mice resulted in neurodegeneration and movement disorder, which were prevented by continuous exposure to 11% oxygen. Specifically, PFF-induced α-syn aggregation resulted in brain tissue hyperoxia, lipid peroxidation and DA neurodegeneration in the SNpc of mice breathing 21% oxygen, but not in those breathing 11% oxygen. This neuroprotective effect of hypoxia was also observed in Caenorhabditis elegans. Moreover, initiating hypoxia 6 weeks after PFF injection reversed motor dysfunction and halted further DA neurodegeneration. These results suggest that hypoxia may have neuroprotective effects downstream of α-syn aggregation in PD, even after symptom onset and neuropathological changes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.K.M. is listed as an inventor on patents filed by MGH on the therapeutic uses of hypoxia. V.K.M. is a paid adviser to 5AM Ventures and Falcon Bio.
Figures




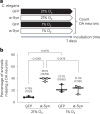



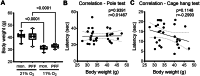
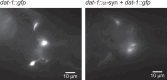
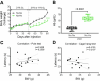

References
-
- Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet397, 2284–2303 (2021). - PubMed
-
- Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain114, 2283–2301 (1991). - PubMed
-
- Schapira, A. H. et al. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet1, 1269 (1989). - PubMed
MeSH terms
Substances
Grants and funding
- T32 GM007753/GM/NIGMS NIH HHS/United States
- K99GM140217/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- T32 GM144273/GM/NIGMS NIH HHS/United States
- 431313887/Deutsche Forschungsgemeinschaft (German Research Foundation)
- T32GM007753/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R56 NS117465/NS/NINDS NIH HHS/United States
- R01 NS124679/NS/NINDS NIH HHS/United States
- T32GM144273/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- K99 GM140217/GM/NIGMS NIH HHS/United States
- R01 NS112373/NS/NINDS NIH HHS/United States
- R01NS124679/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R56NS117465/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

