Chemical autoligation with phosphorothioate- and sulfonamide-terminated DNA via intramolecular cross-activation
- PMID: 40770541
- PMCID: PMC12328621
- DOI: 10.1038/s42004-025-01631-x
Chemical autoligation with phosphorothioate- and sulfonamide-terminated DNA via intramolecular cross-activation
Abstract
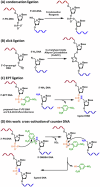
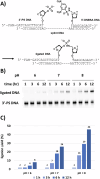
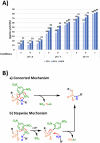
Chemical ligation of oligonucleotides enables assembly of long DNA constructs essential for genome engineering, DNA nanotechnology, and molecular diagnostics, but current methods often require external activators and suffer from reactive intermediate instability. Here we show a reagent-free DNA autoligation strategy based on intramolecular cross-activation between 3'-phosphorothioate (PS) and 5'-dinitrobenzenesulfonamide (DNBSA) termini on a splint DNA, yielding a P3' → N5' phosphoramidate linkage under near-physiological conditions. Ligation proceeds with over 80% yield at 37 °C and pH 8 without external reagents. The DNBSA group exhibits exceptional aqueous stability, and in situ formation of reactive intermediates contributes to high efficiency. This strategy expands the current toolkit for assembling DNA constructs and may facilitate future biotechnological and therapeutic studies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
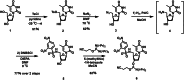

References
-
- Li, X. & Liu, D. R. DNA-templated organic synthesis: nature’s strategy for controlling chemical reactivity applied to synthetic molecules. Angew. Chem. Int. Ed.43, 4848–4870 (2004). - PubMed
-
- Percivalle, C., Bartolo, J.-F. & Ladame, S. Oligonucleotide-templated chemical reactions: pushing the boundaries of a nature-inspired process. Org. Biomol. Chem.11, 16–26 (2013). - PubMed
-
- Gorska, K. & Winssinger, N. Reactions templated by nucleic acids: more ways to translate oligonucleotide-based instructions into emerging function. Angew. Chem. Int. Ed.52, 6820–6843 (2013). - PubMed
-
- Kukwikila, M., Gale, N., El-Sagheer, A. H., Brown, T. & Tavassoli, A. Assembly of a biocompatible triazole-linked gene by one-pot click-DNA ligation. Nat. Chem.9, 1089–1098 (2017). - PubMed
LinkOut - more resources
Full Text Sources

