LRRK2 kinase activity regulates Parkinson's disease-relevant lipids at the lysosome
- PMID: 40770658
- PMCID: PMC12330094
- DOI: 10.1186/s13024-025-00880-7
LRRK2 kinase activity regulates Parkinson's disease-relevant lipids at the lysosome
Abstract
Background: Pathogenic variants in LRRK2 lead to increased kinase activity, and LRRK2 kinase inhibition is being explored in clinical studies as a therapeutic approach for Parkinson's Disease (PD). LRRK2 inhibitors reduce urine levels of bis(monoacylglycerol)phosphate (BMP), a key endolysosomal lipid involved in glycosphingolipid (GSL) catabolism, in preclinical models and clinical subjects. However, how LRRK2 regulates BMP and its significance with respect to lysosomal dysfunction in PD are poorly defined.
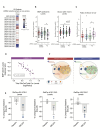
Methods: Using a combination of genetic and pharmacological approaches to modulate LRRK2 kinase activity, we explored the mechanisms by which LRRK2 can regulate the levels of BMP and PD-relevant GSLs across cellular models, including iPSC-derived microglia, and in tissues and biofluids from mice using mass spectrometry. The impact of LRRK2 activity on various aspects of lysosomal function, including endolysosomal GCase activity, was assessed using live-cell imaging and lysosomal immunoprecipitation. We employed imaging mass-spectrometry and FACS-based methods to specifically examine how LRRK2 modulates BMP and GSL levels across different cell types and regions of the brain. To confirm the relevance of our findings to disease, we measured lysosomal biomarkers in urine and cerebrospinal fluid (CSF) from human subjects carrying variants in LRRK2 associated with PD risk and from subjects dosed with a LRRK2 kinase inhibitor.
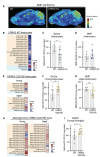
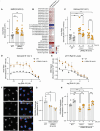
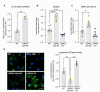
Results: Our data demonstrate that LRRK2 can employ distinct mechanisms to control intracellular BMP levels and modulate lysosomal homeostasis depending on the tissue examined. We show that LRRK2 deletion or inhibition lowers urine BMP levels by reducing the secretion of BMP-containing vesicles from kidney into urine. In other cell types such as microglia, LRRK2-mediated inhibition of β-glucocerebrosidase (GCase), a PD-linked enzyme involved in GSL catabolism, leads to lysosomal GSL accumulation and increases BMP levels as a compensatory response to restore lysosomal homeostasis. LRRK2 inhibition normalizes lysosomal function and reduces GSL levels in preclinical models and CSF from LRRK2-PD patients.
Conclusions: Our study highlights the therapeutic potential of LRRK2 kinase inhibition to improve PD-associated lysosomal dysfunction and supports the utility of GSLs as CSF-based biomarkers of LRRK2 activity.
Trial registration: This work includes results from the following phase 1b study in PD patients: ClinicalTrials.gov ID: NCT03710707; https://clinicaltrials.gov/study/NCT03710707?intr=dnl201&rank=2 . The date of registration was 10/18/2018.
Keywords: BMP and glycosphingolipids; LRRK2; Lysosome; Parkinson’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Study protocols, amendments, and informed consent forms were reviewed and approved by local institutional review boards/independent ethics committees. Written informed consent was obtained from each participant. Consent for publication: Not applicable. Competing interests: The authors declare the following competing interests: M.T.M., X.W., R.G., S.V.A., R.M., S.T.M., M.A., G.A., V.V.B, J.C., C.C., S.S.D., A.C.H., H.N.N., N.E.P., O.B.D., G.D.P., A.A.E., J.V., J.W.L., A.A., J.H.S., S.H.R., and A.G.H. were full time employees and shareholders of Denali Therapeutics during the course of this work. X.W. M.A., A.A.E. and J.D.V. are currently employees of Tenvie Therapeutics.
Figures



References
-
- Abeliovich A, Gitler AD. Defects in trafficking Bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–16. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

