COL6A2 in clear cell renal cell carcinoma: a multifaceted driver of tumor progression, immune evasion, and drug sensitivity
- PMID: 40770761
- PMCID: PMC12330150
- DOI: 10.1186/s12967-025-06793-9
COL6A2 in clear cell renal cell carcinoma: a multifaceted driver of tumor progression, immune evasion, and drug sensitivity
Abstract
Background: Renal Cell Carcinoma (RCC) is a leading cause of cancer-related mortality worldwide, with Clear Cell Renal Cell Carcinoma (ccRCC) comprising 75% of cases. Surgical resection remains the cornerstone of treatment for localized RCC, but its asymptomatic progression and lack of reliable early biomarkers often result in advanced disease at diagnosis. Collagen VI alpha-2 chain (COL6A2), an extracellular matrix protein, has been implicated in tumor progression and metastasis. Despite its established roles in other malignancies, the specific contribution of COL6A2 to ccRCC pathogenesis is poorly understood.
Objective: This study aims to systematically investigate COL6A2 expression in ccRCC, its prognostic value, and its potential impact on the tumor immune microenvironment, cancer stem cell characteristics, and drug response.
Methods: The mRNA and protein expression data for ccRCC were sourced from TCGA, GEO, CPTAC, and ICPC. Single-cell and spatial transcriptomic data were processed using Seurat with quality control measures. Clinical correlations and survival analyses, including immune infiltration and COL6A2 expression, were assessed using Cox regression and Kaplan-Meier curves. Cancer stemness was evaluated using six stemness indices. Differential expression and pathway analyses (GO, KEGG, GSEA) were performed with DESeq2 and clusterProfiler. Drug sensitivity and immunotherapy response were predicted using GDSC, CTRP, and TIDE databases. Functional studies, including colony formation and invasion assays, as well as in vivo xenograft models, assessed the impact of COL6A2 on tumor progression and therapy response.
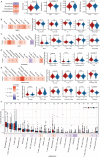
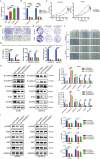
Results: COL6A2 expression was significantly upregulated in ccRCC compared to normal tissues. High COL6A2 expression correlated with poorer overall survival (OS), progression-free interval (PFI), and progression-free survival (PFS), establishing it as an independent prognostic factor for ccRCC. Additionally, COL6A2 expression was positively associated with immune-suppressive cell infiltration, suggesting an immunosuppressive tumor microenvironment. COL6A2 was also linked to enhanced stem cell-like properties, invasiveness, and metastatic potential. Pathway enrichment analyses revealed that COL6A2 may influence tumor progression by regulating the epithelial-mesenchymal transition (EMT) process and activating the PI3K-Akt signaling pathway. Notably, high COL6A2 expression correlated with enhanced responsiveness to sunitinib but resistance to immunotherapy, highlighting its dual role in therapy selection.
Conclusion: This study identifies COL6A2 as a powerful prognostic biomarker and a driver of ccRCC progression through EMT and immune suppression. Targeting COL6A2 holds promise for improving immunotherapy efficacy and personalizing treatment strategies, offering new hope for ccRCC patients facing limited options.
Keywords: COL6A2; Pharmacological prediction; Prognostic significance; Renal cancer; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: For animal study: This study was approved by the Medical Ethics Committee of Zhongnan Hospital, Wuhan University (approval MRI2024-LAC209). For human study: not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

