Transcriptional and post-transcriptional responses to amyloid- β in cerebral amyloid angiopathy
- PMID: 40770922
- PMCID: PMC12331648
- DOI: 10.1177/0271678X251366082
Transcriptional and post-transcriptional responses to amyloid- β in cerebral amyloid angiopathy
Abstract
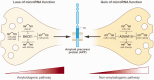
Cerebral amyloid angiopathy (CAA) is a common age-related small vessel disease characterized by amyloid-beta (Aβ) accumulation in cortical and leptomeningeal blood vessel walls. Reduced Aβ clearance in the vasculature elevates the risk of CAA, while increasing evidence indicates that enhanced Aβ production in neurons also contributes. The impact of Aβ on the diverse cell types of the neurovascular unit (NVU)-including endothelial cells (ECs), pericytes, neurons, vascular smooth muscle cells (VSMCs), and astrocytes-remains unclear. This narrative review proposes that Aβ accumulation in NVUs during CAA drives a transcriptional response that reduces Aβ clearance while activating a neuron-specific post- transcriptional response that enhances Aβ production. Specifically, Aβ in NVUs was found to initiate a transcriptional cascade that destabilizes endothelial cells, increases blood-brain barrier permeability, and damages pericytes, ultimately inducing inflammatory and dysfunctional changes in VSMCs. These changes cause mitochondrial dysfunction and TGFβ deregulation in neurons, activating profibrotic mechanisms. Post-transcriptional regulation by microRNA networks in neurons affects Aβ processing by controlling the balance between amyloidogenic and non-amyloidogenic pathways through BACE1 and ADAM10 expression respectively. This review improves our understanding of Aβ accumulation in neurovascular units in CAA, potentially leading to better diagnostics, early biomarkers, and tools for prognosis and treatment.
Keywords: Cerebral amyloid angiopathy; amyloid-beta; microRNA; neurovascular unit; transcripts.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Pericyte loss: a key factor inducing brain Aβ40 accumulation and neuronal degeneration in cerebral amyloid angiopathy.Exp Brain Res. 2025 Aug 1;243(8):191. doi: 10.1007/s00221-025-07134-4. Exp Brain Res. 2025. PMID: 40748498
-
Human iPSC-derived pericyte-like cells carrying APP Swedish mutation overproduce beta-amyloid and induce cerebral amyloid angiopathy-like changes.Fluids Barriers CNS. 2024 Sep 27;21(1):78. doi: 10.1186/s12987-024-00576-y. Fluids Barriers CNS. 2024. PMID: 39334385 Free PMC article.
-
Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy.Mol Neurodegener. 2023 Mar 16;18(1):17. doi: 10.1186/s13024-023-00610-x. Mol Neurodegener. 2023. PMID: 36922879 Free PMC article.
-
Dysfunction of the blood-brain barrier in Alzheimer's disease: Evidence from human studies.Neuropathol Appl Neurobiol. 2022 Apr;48(3):e12782. doi: 10.1111/nan.12782. Epub 2022 Feb 2. Neuropathol Appl Neurobiol. 2022. PMID: 34823269
-
Using Neuroimaging to Study Cerebral Amyloid Angiopathy and Its Relationship to Alzheimer's Disease.J Alzheimers Dis. 2024;97(4):1479-1502. doi: 10.3233/JAD-230553. J Alzheimers Dis. 2024. PMID: 38306032 Review.
References
-
- Koemans EA, Chhatwal JP, van Veluw SJ, et al. Progression of cerebral amyloid angiopathy: a pathophysiological framework. Lancet Neurol 2023; 22: 632–642. - PubMed
-
- Attems J, Lintner F, Jellinger KA. Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and alzheimer disease pathology. Acta Neuropathol 2004; 107: 283–291. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

