Partially hydrolyzed, whey-based infant formula with six human milk oligosaccharides, B. infantis LMG11588, and B. lactis CNCM I-3446 is safe, well tolerated, and improves gut health: a staged analysis of a randomized trial
- PMID: 40771223
- PMCID: PMC12325064
- DOI: 10.3389/fnut.2025.1628847
Partially hydrolyzed, whey-based infant formula with six human milk oligosaccharides, B. infantis LMG11588, and B. lactis CNCM I-3446 is safe, well tolerated, and improves gut health: a staged analysis of a randomized trial
Erratum in
-
Correction: Partially hydrolyzed, whey-based infant formula with six human milk oligosaccharides, B. infantis LMG11588, and B. lactis CNCM I-3446 is safe, well tolerated, and improves gut health: a staged analysis of a randomized trial.Front Nutr. 2025 Sep 12;12:1676657. doi: 10.3389/fnut.2025.1676657. eCollection 2025. Front Nutr. 2025. PMID: 41019559 Free PMC article.
Abstract
Background and aims: Gut health and microbiome development are closely linked in early life, with human milk oligosaccharides (HMOs) playing a key role. This study reports results through 4 months of age from a trial evaluating an infant formula containing a synbiotic blend of HMOs and probiotics, focusing on growth, gastrointestinal (GI) tolerance, and gut health biomarkers from birth to 15 months.
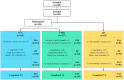
Materials and methods: Healthy infants aged ≤14 days were randomized to receive either the experimental formula (SYN; control formula supplemented with six HMOs and two probiotics [B. infantis, B. lactis]) or the control formula (CTRL; partially hydrolyzed 100% whey-based formula). A non-randomized breastfed (BF) group served as a reference. The primary endpoint was weight gain velocity in SYN vs. CTRL through 4 months of age. Secondary endpoints included fecal outcomes (abundance of bifidobacteria, immune and gut health markers), GI tolerance, and adverse events (AEs).
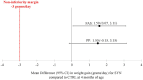
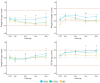
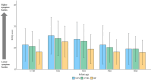
Results: The full analysis set (FAS) included 313 infants (118 in SYN, 114 in CTRL, and 81 BF), while the per-protocol population (PP) included 227 infants (84 in SYN, 84 in CTRL, and 59 BF). Weight gain velocity through 4 months in the SYN group was non-inferior to that in the CTRL group in both FAS and PP analyses (both p < 0.0001). Parent-reported GI tolerance and stool patterns were similar between SYN and CTRL groups through 4 months. At 3 months, Bifidobacteria abundance was significantly higher in the SYN group compared to the CTRL group (p = 0.004). Fecal pH was lower in the SYN group than in the CTRL group (p = 0.018) and more closely resembled that of the BF group. Immune and gut health markers were similar between the SYN and BF groups. No significant differences in AEs were observed across groups.
Conclusion: The synbiotic-supplemented infant formula supported healthy, age-appropriate growth, good GI tolerance, and increased the abundance of beneficial bifidobacteria through 4 months of age.
Clinical trial registration: https://clinicaltrials.gov/study/NCT04962594.
Keywords: bifidobacteria; gastrointestinal tolerance; growth; gut health; microbiota.
Copyright © 2025 Picaud, Claris, Gil-Campos, De La Cueva, Cornette, Alliet, Léké, Castanet, Piloquet, de Halleux, Mitanchez, Vandenplas, Maton, Jochum, Olbertz, Policarpo, Lavalle, Fumero, Rodriguez-Garcia, Moll, Silva-Zolezzi, Zemrani, Hays, Sprenger and Miranda-Mallea.
Conflict of interest statement
Authors LL, CF, ISZ, BZ, NPH, and NS are employed by Société des Produits Nestlé S.A. Author JM Moll is employed by Cmbio, and author PRG was employed by Cmbio at the time of completion of this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Storm HM, Shepard J, Czerkies LM, Kineman B, Cohen SS, Reichert H, et al. 2′-fucosyllactose is well tolerated in a 100% whey, partially hydrolyzed infant formula with Bifidobacterium lactis: a randomized controlled trial. Glob Pediatr Health. (2019) 6:2333794x19833995. doi: 10.1177/2333794X19833995, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

