Postbiotics enhance the efficacy of derivative compound mouthwash against clinical Helicobacter pylori strains
- PMID: 40771311
- PMCID: PMC12325292
- DOI: 10.3389/fcimb.2025.1629106
Postbiotics enhance the efficacy of derivative compound mouthwash against clinical Helicobacter pylori strains
Abstract
Background: A previous study indicated that poly L-lysine-glycerol monolaurate mouthwash reduced the virulence of Helicobacter pylori; however, these compounds are derivatives. Thus, this study aimed to compare the effects of postbiotics, postbiotic-glycerol monolaurate, and poly L-lysine-glycerol monolaurate mouthwashes against clinical H. pylori strains.
Methods: Postbiotics, Lacticaseibacillus paracasei SD1, L. rhamnosus SD4, and L. rhamnosus SD11 were examined for anti-bacterial activity and synergistic effects. Subsequently, mouthwashes containing postbiotics, postbiotic-glycerol monolaurate, and poly L-Lysine-glycerol monolaurate were prepared and evaluated for their ability to reduce H. pylori adhesion to host cells, suppress inflammation induced by H. pylori, eradicate biofilm, decrease cagA expression, and assess epithelial cell viability. The stability of the mouthwashes was evaluated every 4 weeks up to 24 weeks for their efficacy against H. pylori growth, biofilm eradication, and epithelial cell viability.
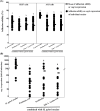
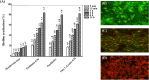
Results: The postbiotics, L. paracasei SD1 and L. rhamnosus SD11, demonstrated significant anti-H. pylori activity, with synergistic effects observed in combinations with derivative compounds. Postbiotic-glycerol monolaurate mouthwashes exhibited higher efficacy in reducing H. pylori adhesion to host cells (42.64-43.83%), suppressing pro-inflammatory cytokines, eradicating biofilm (82.62% at 24 h), and reducing cagA expression (112.60 fold) compared to others. Such mouthwashes also displayed low cytotoxicity (< 30% for 15 min) to all cells tested. The stability was observed up to 24 weeks.
Conclusion: This in vitro study demonstrated that postbiotic-glycerol monolaurate mouthwash revealed the highest efficacy against H. pylori with low cytotoxicity to host cells. The stability lasted for 24 weeks.
Keywords: Helicobacter pylori; derivative compound; glycerol monolaurate; mouthwash; postbiotics.
Copyright © 2025 Teanpaisan and Pahumunto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Azizimoghaddam Y., Kermanpour S., Mirzaei N., Houri H., Nabavi-Rad A., Aghdaei H. A., et al. (2023). Genetic diversity of Helicobacter pylori type IV secretion system cagI and cagN genes and their association with clinical diseases. Sci. Rep. 13, 10264. doi: 10.1038/s41598-023-37392-7, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

