Prevention of donation-related infections: investigating the use of antibiotics in the decontamination of preservation fluid for organ transplantation
- PMID: 40771313
- PMCID: PMC12325265
- DOI: 10.3389/fcimb.2025.1572799
Prevention of donation-related infections: investigating the use of antibiotics in the decontamination of preservation fluid for organ transplantation
Abstract
Background: Donation-related infections (DRIs), particularly those caused by carbapenem-resistant gram-negative bacteria (CRGNB), can have disastrous consequences because of their extensive drug resistance. Contamination during graft acquisition and transport can lead to DRIs, and the use of antibiotics in preservation fluid (PF) before organ transplantation can reduce the incidence of DRIs. This study was to determine and compare the effectiveness of different PF decontamination regimens to prevent CRGNB related DRIs.
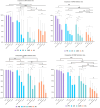
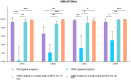
Methods: Twelve CRGNB strains were chosen to be the targets of decontamination, and a drug concentration gradient was established for each test drug based on the previous clinical research. In addition the standard decontamination procedures were performed to evaluate the antimicrobial effectiveness of polymyxin B (PB), colistin sulfate (CS), colistimethate sodium (CMS) and amikacin (AK) in the 0~4°C PF, and to explore the antimicrobial effects of CMS after different preprocessing methods.
Results: PB and CS exhibited significantly better antimicrobial effectiveness against CRGNB than AK and CMS in the 0~4°C PF, and the antimicrobial effects on CRGNB increased with the increasing concentration of drugs. Notably, CMS after pretreatment (CMS-AP), its antibacterial was significantly enhanced at 4°C.
Conclusions: The PF decontamination is important in preventing the DRIs caused by CRGNB, and the decontamination regimens based on PB or CS were confirmed effective. Notably, CMS could even achieve a better decontamination effect than PB after a simple and fast pretreatment.
Keywords: CRGNB; decontamination; donation-related infection; polymyxin; transplant.
Copyright © 2025 Duan, Yu, Zhang, Li, Chen, Li, Wan, Wu, Zeng, Li and Sui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ahumada Topete V. H., de Dios Sanchez K. J., Casas Aparicio G. A., Hernandez Silva G., Lopez Vejar C. E., Torres Espíndola L. M., et al. (2023). Adverse events and drug resistance in critically ill patients treated with colistimethate sodium: A review of the literature. Infect. Drug Resist. 16, 1357–1366. doi: 10.2147/IDR.S398930, PMID: - DOI - PMC - PubMed
-
- Corbel A., Ladrière M., Le Berre N., Durin L., Rousseau H., Frimat L., et al. (2020). Microbiological epidemiology of preservation fluids in transplanted kidney: a nationwide retrospective observational study. Clin. Microbiol. Infect. 26, 475–484. doi: 10.1016/j.cmi.2019.07.018, PMID: - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

