Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- PMID: 40771412
- PMCID: PMC12326303
- DOI: 10.1515/biol-2025-1129
Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
Abstract
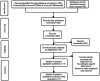
This article conducts a meta-analysis to evaluate the safety and efficacy of PD-1/PD-L1 inhibitors in patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). A total of 63 papers were initially retrieved, and eight clinical studies were collected. The estimated effect of ORR was [OR = 0.40, 95% CI 0.29-0.51; p = 0.08], the estimated effect of complete response rate was [OR = 0.21, 95% CI 0.14-0.31; p < 0.001], while the estimated effect of 1-year progression-free survival was [OR = 0.33, 95% CI 0.22-0.47; p = 0.01]. The estimated effect of 1-year OS was [OR = 0.67, 95% CI 0.55-0.77; p = 0.05]. In addition, the estimated effect of grade 3 adverse events was [OR = 0.33, 95% CI 0.22-0.46; p = 0.01]. Overall, PD-1/PD-L1 inhibitors demonstrated suboptimal therapeutic efficacy in the selected trials for R/R DLBCL. However, combining PD-1/PD-L1 inhibitors with CAR-T showed potential for improved treatment outcomes. Additionally, PD-1/PD-L1 inhibitors were found to be safe and well-tolerated in patients with R/R DLBCL.
Keywords: PD-1/PD-L1 blockade; immune checkpoint molecule; meta-analysis; relapsed/refractory diffuse large B-cell lymphoma; treatment.
© 2025 the author(s), published by De Gruyter.
Conflict of interest statement
Conflict of interest: Authors state no conflict of interest.
Figures




References
-
- Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2016;52:216–21. 10.1038/bmt.2016.213. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
