Risk Prediction and Management of BKPyV-DNAemia in Kidney Transplant Recipients: A Multicenter Analysis of Immunosuppressive Strategies
- PMID: 40771589
- PMCID: PMC12327399
- DOI: 10.3389/ti.2025.14738
Risk Prediction and Management of BKPyV-DNAemia in Kidney Transplant Recipients: A Multicenter Analysis of Immunosuppressive Strategies
Abstract
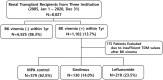
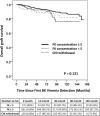
BK polyomavirus (BKPyV) DNAemia remains a major complication in kidney transplantation (KT), requiring nuanced adjustments to immunosuppressive regimens to control viral replication while minimizing rejection risk. This retrospective multicenter cohort study included 8,027 KT recipients, of whom 1,102 developed BKPyV-DNAemia within the first year. Among them, 927 patients with complete therapeutic drug monitoring (TDM) data were categorized into three groups based on post- BKPyV-DNAemia immunosuppressive strategies: mycophenolic acid (MPA) control, sirolimus, and leflunomide. Multivariate logistic regression and Cox analyses identified risk factors for BKPyV-DNAemia treatment failure, acute rejection, and graft loss. Tacrolimus trough levels below 5 ng/mL and complete withdrawal of calcineurin inhibitors (CNIs) significantly increased rejection risk (OR = 2.65, P = 0.033). Maintaining tacrolimus levels between 5 and 7 ng/mL was associated with optimal viral control and lower rejection rates. Leflunomide substitution reduced BKPyV burden but increased rejection risk (OR = 2.14, P < 0.001). Sirolimus-based regimens with CNI withdrawal led to the highest rejection risk (OR = 6.00, P = 0.044) and a trend toward increased graft failure (HR = 4.37, P = 0.07). A tacrolimus target of ≥5 ng/mL emerged as optimal for balancing BKPyV-DNAemia suppression and long-term graft survival. While leflunomide is effective for viral control, its immunological risks warrant careful patient selection and monitoring.
Keywords: Bk virus; calcineurin inhibitor; immunosuppressive therapy; kidney transplantation; tacrolimus trough level.
Copyright © 2025 Kim, Kwon, Han, Ko, Shin, Kim, Lee, Park, Kwon and Min.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, et al. Kidney Transplantation as Primary Therapy for End-Stage Renal Disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) Conference. Clin J Am Soc Nephrol (2008) 3(2):471–80. 10.2215/CJN.05021107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

