Influence of "cryoconcentration" on the composition of bacterial communities in semi-enclosed shallow water lakes
- PMID: 40771679
- PMCID: PMC12325427
- DOI: 10.3389/fmicb.2025.1623773
Influence of "cryoconcentration" on the composition of bacterial communities in semi-enclosed shallow water lakes
Abstract
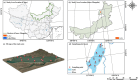
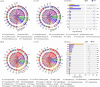
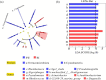
Bacteria serve as vital indicators of the lake ecosystem health. Although substantial progress has been made in investigating the structural features of lake bacterial communities, limited attention has been paid to the dynamic assembly processes and driving factors affecting bacterial communities in ice and water environments during the freeze-up period. In this study, we investigated aggregation and compositional changes in bacterial communities in the internal ice-covered state of Lake Hulun. We examined the effects of cryoconcentration under low-temperature conditions on community assembly and systematically analyzed the physicochemical parameters as well as α- and β-diversity of bacterial communities in bottom ice (BI) and surface water (SW) media. Bacterial diversity was significantly higher in SW than in BI. Among the dominant taxa, eight phyla were shared between both environments. Firmicutes and Patescibacteria were dominant in the BI, whereas Gemmatimonadota and Bdellovibrionota were dominant in the SW. Nutrient transport driven by cryoconcentration emerged as a key factor influencing bacterial community assembly. Specifically, total nitrogen and salinity regulated the balance between stochastic and deterministic processes in BI and SW, respectively. Overall, the distinct environmental conditions of BI and SW weakened the diffusion capacity of bacterial communities, resulting in diffusion-limited and drift-dominated assembly processes. These findings offer new insights into the mechanisms underlying bacterial interactions and community assembly in ice-covered lake habitats and provide a scientific foundation for the management and preservation of lake ecosystems under ice-covered conditions.
Keywords: Assembly process; bacterial community structure; co-occurrence network; freezing process; ice-water interface.
Copyright © 2025 Bingxian, Yujiao, Wenbao and Hengshuai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bramburger A. J., Ozersky T., Silsbe G. M., Crawford C., Olmanson L., Shchapov K. (2023). The not-so-dead of winter: Underwater light climate and primary productivity under snow and ice cover in inland lakes. Inland Waters 13 1–12. 10.1080/20442041.2022.2102870 - DOI
-
- Cai Z. S., Jin T. Y., Li C. Y., Ofterdinger U., Zhang S. (2016). Is China’s fifth-largest inland lake to dry-up? Incorporated hydrological and satellite-based methods for forecasting Hulun lake water levels. Adv. Water. Resour. 94 185–199. 10.1016/j.advwatres.2016.05.010 - DOI
LinkOut - more resources
Full Text Sources

