Advances in adhesion-related pathogenesis in Mycoplasma pneumoniae infection
- PMID: 40771685
- PMCID: PMC12325244
- DOI: 10.3389/fmicb.2025.1613760
Advances in adhesion-related pathogenesis in Mycoplasma pneumoniae infection
Abstract
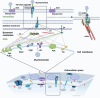
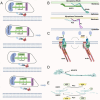
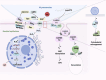
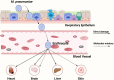
Mycoplasma pneumoniae is a leading cause of community-acquired pneumonia (CAP) and upper respiratory tract infections, particularly in children and immunocompromised individuals. The growing global prevalence of macrolide-resistant M. pneumoniae (MRMP) further emphasizes the urgent need to elucidate its pathogenic mechanisms. Among these, adhesion plays a central role, serving as a prerequisite for colonization and disease progression, and thus warrants detailed investigation. The terminal organelle of M. pneumoniae mediates both adhesion and gliding motility, facilitating colonization, tissue invasion, and potential systemic spread. In the lung, adhesion triggers cytotoxic effects through the release of hydrogen peroxide (H2O2) and CARDS toxin (CARDS TX), promotes excessive inflammatory responses, and enables immune evasion via antigenic variation. Extrapulmonary manifestations may also arise either from direct bacterial dissemination or autoimmune responses induced by molecular mimicry between bacterial and host antigens. In addition, recent advances suggest that therapies and vaccines directed at the adhesion mechanism of M. pneumoniae may offer promising strategies for combating MRMP infections. Although progress has been made, the adhesion-related pathogenesis of M. pneumoniae, as well as the prospects for therapies and vaccines targeting this mechanism, remains incompletely defined. This review synthesizes current insights into adhesion-mediated mechanisms and highlights emerging therapeutic strategies targeting adhesion, aiming to support more effective treatment and prevention of M. pneumoniae infection.
Keywords: M. pneumonia; adhesion; terminal organelle; treatment; vaccines.
Copyright © 2025 Sun, Ling, Li, Ma, Jie, Luo, Li, Yin, Wang, Meng and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

