CD7 CAR-T therapy: current developments, improvements, and dilemmas
- PMID: 40771729
- PMCID: PMC12327587
- DOI: 10.1097/BS9.0000000000000247
CD7 CAR-T therapy: current developments, improvements, and dilemmas
Abstract
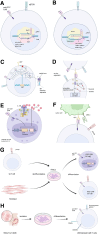
Chimeric antigen receptor (CAR) T-cell therapy is an epoch-making immunotherapy for the treatment of relapsed or refractory (r/r) blood tumors, as demonstrated by its successful implementation in r/r B cell-derived malignancies. However, replicating this success in T-cell leukemia or lymphoma remains challenging. Among the various potential target antigens, CD7 has garnered attention as a promising candidate. CD7 CAR-T therapy is one of the most extensively studied approaches for treating r/r T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/LBL) and r/r acute myeloid leukemia (AML). Based on the source of T cells, CAR-T products can be categorized as autologous and allogeneic, both of which are being tested in clinical trials, each offering specific advantages. Allogeneic CD7 CAR-T cells outperform autologous cells in terms of reducing manufacturing costs, ensuring consistent quality, and improving affordability and availability. Despite these advantages, challenges like graft-versus-host disease (GVHD), host-versus-graft reaction (HVGR), and fratricide pose significant barriers to the clinical application of allogeneic CD7 CAR-T cells. However, innovative gene-editing techniques, such as CRISPR/Cas9 and base editing, and more promising cell sources, such as natural killer T (NKT) cells and induced pluripotent stem cells (iPSCs), are emerging as potential solutions. In this review, we discuss the different categories of CD7 CAR-T products, their application in clinical settings, and directions for refinement.
Keywords: AML; CAR-T; CD7; GVHD; clinical application; fratricide; r/r T-ALL/LBL.
Copyright © 2025 The Authors. Published by Wolters Kluwer Health Inc., on behalf of the Chinese Medical Association (CMA) and Institute of Hematology, Chinese Academy of Medical Sciences & Peking Union Medical College (IHCAMS).
Figures
References
-
- van Dongen JJ, Quertermous T, Bartram CR, et al. T cell receptor-CD3 complex during early T cell differentiation. Analysis of immature T cell acute lymphoblastic leukemias (T-ALL) at DNA, RNA, and cell membrane level. J Immunol 1987;138(4):1260–1269. - PubMed
-
- Campana D, Thompson JS, Amlot P, Brown S, Janossy G. The cytoplasmic expression of CD3 antigens in normal and malignant cells of the T lymphoid lineage. J Immunol 1987;138(2):648–655. - PubMed
-
- Shah B, Mattison RJ, Abboud R, et al. Acute lymphoblastic leukemia, version 2.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2024;22(8):563–576. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
