Tracing the development of CAR-T cell design: from concept to next-generation platforms
- PMID: 40771804
- PMCID: PMC12326545
- DOI: 10.3389/fimmu.2025.1615212
Tracing the development of CAR-T cell design: from concept to next-generation platforms
Abstract
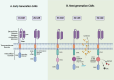
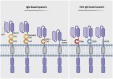
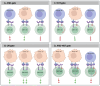
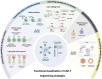
Chimeric Antigen Receptor (CAR)-T cell therapy represents a transformative breakthrough in cancer immunotherapy by harnessing the adaptive immune system to selectively eradicate cancer cells. Pioneering advances in the treatment of hematological malignancies have led to the FDA approval of several CAR-T cell therapies, particularly for patients with relapsed or refractory disease. This success is a result of continuous refinements in CAR architecture, which have evolved from early prototypes with limited therapeutic efficacy to advanced next-generation receptors that incorporate co-stimulatory domains, cytokine signaling, safety switches, and precision control mechanisms. This review elucidates the fundamental rationale behind CAR-T cell development and addresses key biological challenges encountered. Advances in receptor engineering, metabolic reprogramming, and optimized immune signaling have markedly enhanced the persistence, antitumor activity, and safety profiles of CAR-T cells. Additionally, emerging genetic engineering tools, including CRISPR, base editing, prime editing, and RNA and epigenome editing, hold promise for reducing immunogenicity and minimizing the risk of graft-versus-host disease (GVHD). However, CAR-T cell therapy continues to face several challenges, including severe side effects such as cytokine release syndrome (CRS) and neurotoxicity, inconsistent therapeutic responses, and high production costs. To overcome these barriers, novel approaches are under development that include generating CAR-T cells in vivo, utilizing logic-gated CAR systems, and expanding CAR platforms to include other immune effector cells, such as natural killer cells (CAR-NK) and macrophages (CAR-M). The future of CAR-based therapies is expected to integrate synthetic biology, immune checkpoint modulation, and innovative delivery methods to enhance both therapeutic efficacy and safety. This review synthesizes current knowledge and emerging strategies to guide future advancements aimed at expanding the applicability of CAR therapy to various cancer types and potentially other diseases.
Keywords: CAR design; CAR-T cell therapy; T cell engineering; adoptive cell therapy; gene editing; immunotherapy; next-generation CAR; synthetic immunology.
Copyright © 2025 Alsaieedi and Zaher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548, PMID: - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

