Immunosuppressive cells in acute myeloid leukemia: mechanisms and therapeutic target
- PMID: 40771826
- PMCID: PMC12325080
- DOI: 10.3389/fimmu.2025.1627161
Immunosuppressive cells in acute myeloid leukemia: mechanisms and therapeutic target
Abstract
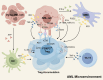
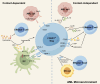
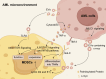
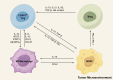
Immunotherapy has emerged as a cornerstone strategy for augmenting therapeutic efficacy in acute myeloid leukemia (AML). The immunosuppressive AML microenvironment, characterized by profound immune dysfunction, critically impairs anti-leukemic immune surveillance. This immunologically hostile niche is principally governed by specialized immunosuppressive cell populations-notably regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), leukemia-associated macrophages (LAMs), and regulatory B cells (Bregs)-which collectively establish an immune-privileged sanctuary for leukemic cells. This review critically examines three fundamental aspects of these immunosuppressive regulators in AML pathogenesis: (1) their recruitment dynamics within the leukemic niche, (2) the molecular mechanisms underlying their immunosuppressive functions, and (3) current and emerging therapeutic approaches designed to neutralize their inhibitory effects. Through this comprehensive analysis, we aim to provide a mechanistic framework for developing more effective immunotherapeutic interventions against AML.
Keywords: acute myeloid leukemia; leukemia-associated macrophages; leukemia-associated neutrophils; myeloid-derived suppressor cells; regulatory B cells; regulatory T cells.
Copyright © 2025 Liu, Yang, Qi, Ma, Guo, Guo, Liu, Liu, Xiao and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. (2020) 13:72. doi: 10.1186/s13045-020-00908-z, PMID: - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

