The Hypomethylating Agent 5-Azacitidine Potentiates the Effect of RAS and Sp1 Inhibitors in Neuroblastoma Cells
- PMID: 40771851
- PMCID: PMC12322886
- DOI: 10.32607/actanaturae.27558
The Hypomethylating Agent 5-Azacitidine Potentiates the Effect of RAS and Sp1 Inhibitors in Neuroblastoma Cells
Abstract
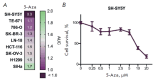
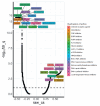
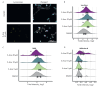
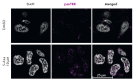
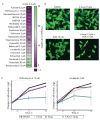
Neuroblastoma is a malignant solid tumor caused by the transformation of neural crest cells. Neuroblastoma predominantly occurs in children and is associated with a poor prognosis. In this regard, the development of novel approaches to neuroblastoma treatment, including combination therapy, is relevant. DNA hypermethylation of neuroblastoma cells indicates that it is possible to use hypomethylating agents in a combination therapy of the disease. In order to identify effective combinations of antitumor drugs, we analyzed the transcriptomic changes that take place in neuroblastoma SH-SY5Y cells after treatment with the hypomethylating agent 5-azacitidine and then experimentally tested the effectiveness of these combinations. Mithramycin A and lonafarnib were the two drugs that, in combination with 5-azacitidine, appeared to exert a synergistic effect on SH-SY5Y cell death. These drugs inhibit the signaling pathway associated with the transcription factor Sp1 and RAS-MAPK signaling pathway, which are activated by 5-azacitidine. An analysis of the signaling pathways also revealed an activation of the signaling pathways associated with neuroblastoma cell differentiation, as well as apoptosis induction, as confirmed by multiplex and confocal microscopy. Hence, by analyzing the changes in the signaling pathways, the mechanisms of cell death and cell adaptation to hypomethylating agents can be understood, and this can be further used to develop novel therapeutic approaches to neuroblastoma therapy.
Keywords: combination therapy; epigenetic regulators; pediatric malignant diseases.
Copyright ® 2025 National Research University Higher School of Economics.
Figures


Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.Cochrane Database Syst Rev. 2015 Oct 5;2015(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. Cochrane Database Syst Rev. 2015. PMID: 26436598 Free PMC article.
-
High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.Cochrane Database Syst Rev. 2013 Aug 22;(8):CD006301. doi: 10.1002/14651858.CD006301.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Oct 05;(10):CD006301. doi: 10.1002/14651858.CD006301.pub4. PMID: 23970444 Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L., Weiss W.A., Nat. Rev. Dis. Prim. 2016;2(1):16078.:10.1038/nrdp.2016.78. - PubMed
-
- Krystal J., Foster J.H., Children. 2023;10(8):1302.:10.3390/children10081302.
-
- Straathof K., Flutter B., Wallace R., Jain N., Loka T., Depani S., Wright G., Thomas S., Cheung G.W.-K., Gileadi T., Sci. Transl. Med. 2020;12(571):10.1126/scitranslmed.abd6169. - PubMed
LinkOut - more resources
Full Text Sources
