Various Configurations of Au@Pt Nanostructures on Modified Electrochemical Sensors for H2O2 Detection
- PMID: 40771857
- PMCID: PMC12322878
- DOI: 10.1021/acsanm.5c03116
Various Configurations of Au@Pt Nanostructures on Modified Electrochemical Sensors for H2O2 Detection
Abstract
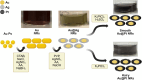
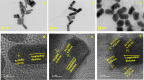
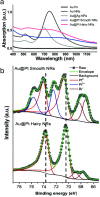
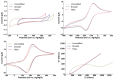
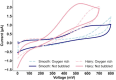
Hydrogen peroxide (H2O2) is a vital metabolite involved in numerous biological processes, with physiological concentrations in humans ranging from 1 to 50 μM. Its rapid production, utilization, and decomposition make accurate low-concentration detection challenging. Although precious metals such as gold and platinum are effective for H2O2 detection, their high cost and limited availability necessitate alternative strategies. Nanostructuring these materials into core-shell nanorods (their size ∼ 40 nm in length) offers a sustainable, efficient solution by reducing material usage while enhancing performance. In this study, we modified glassy carbon electrodes with two types of Au@Pt nanorods (NR) for H2O2's cyclic voltammetric and chronoamperometric detection: plain-surfaced (Smooth) and appendaged-surfaced (Hairy). Both sensors exhibit rapid stabilization, achieving reliable measurements within 5 s, suitable for capturing the volatile nature of H2O2. The Hairy NRs demonstrate superior performance, attributed to the increased presence of catalytically active Pt(0) compared to the less active Pt-(II) in Smooth NRs. This difference in oxidation states, combined with the enhanced surface geometry of Hairy NRs, results in faster kinetics, a wider linear detection range (500 nM-50 μM vs 1-50 μM), lower detection limit (189 nM vs 370 nM), and nearly double sensitivity. To simulate physiological conditions, we assessed oxygen interference and evaluated performance in biologically relevant environments. Cell viability tests were conducted to determine the nanoparticles' toxicity toward neuroblastic cells. These findings support further development of modified Au@Pt nanorod electrodes for in vivo and in vitro applications. With rapid response times, favorable detection limits, and high sensitivity, these sensors are promising for biomedical diagnostics, environmental monitoring, and studying neurotransmitters like glutamate.
Keywords: Au@Pt nanorods; Pt oxidation state; cell toxicity assay; electrochemical sensor; hydrogen peroxide (H2O2); nanoparticle fabrication; surface chemistry.
© 2025 The Authors. Published by American Chemical Society.
Figures



Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Carbon dioxide detection for diagnosis of inadvertent respiratory tract placement of enterogastric tubes in children.Cochrane Database Syst Rev. 2025 Feb 19;2(2):CD011196. doi: 10.1002/14651858.CD011196.pub2. Cochrane Database Syst Rev. 2025. PMID: 39968844
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
LinkOut - more resources
Full Text Sources
