This is a preprint.
SPEECHLESS duplication in grasses expands potential for environmental regulation of stomatal development
- PMID: 40771893
- PMCID: PMC12327695
- DOI: 10.1101/2025.07.29.667563
SPEECHLESS duplication in grasses expands potential for environmental regulation of stomatal development
Abstract
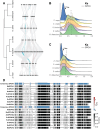
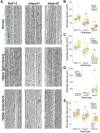
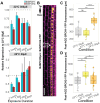
Plants acquire atmospheric carbon dioxide for photosynthesis while minimizing water loss and do so by regulating stomatal function and development. The ancestral basic helix-loop-helix transcription factor (TF) gene that drove stomata production in early land plants diversified in sequence and function to become paralogs SPEECHLESS (SPCH), MUTE, and FAMA. Extant angiosperms use these three TFs and their heterodimer partners to regulate stomatal cell identities. Grasses exhibit a particularly interesting set of duplications and losses of SPCH. Using phylogenetic methods, we tracked the duplication of SPCH to the Poaceae-specific rho whole genome duplication and demonstrated that both paralogs remain under selection. By following responses to environmental change in B. distachyon plants bearing mutations in either BdSPCH1 or BdSPCH2, we reveal paralog-specific divergence in response to light or temperature shifts, and further show this behavior is conserved O. sativa SPCH paralogs. Plausible molecular mechanisms underpinning paralog divergence, and cellular mechanisms driving the stomatal phenotypes are supported by analyses of RNA and protein expression in B. distachyon and sequence variation among grasses. These studies suggest ways in which a duplication of a key stomatal regulator enables adaptation and could inform genetic strategies to mitigate anticipated stressors in agronomically important plants.
Keywords: Poaceae; SPEECHLESS; Stomata; WGD; evolution; gene duplication; subfunctionalization.
Figures

Similar articles
-
Direct Control of SPEECHLESS by PIF4 in the High-Temperature Response of Stomatal Development.Curr Biol. 2018 Apr 23;28(8):1273-1280.e3. doi: 10.1016/j.cub.2018.02.054. Epub 2018 Apr 5. Curr Biol. 2018. PMID: 29628371 Free PMC article.
-
Reduced stomatal density improves water-use efficiency in grapevine under climate scenarios of decreased water availability.Plant Cell Rep. 2025 Aug 7;44(9):195. doi: 10.1007/s00299-025-03577-9. Plant Cell Rep. 2025. PMID: 40775479 Free PMC article.
-
Warm temperature modifies cell fates to reduce stomata production in Arabidopsis.New Phytol. 2025 Jul 26. doi: 10.1111/nph.70396. Online ahead of print. New Phytol. 2025. PMID: 40716027
-
The PEAPOD repressor complex in Arabidopsis stomatal development.Front Plant Sci. 2025 Jul 23;16:1641102. doi: 10.3389/fpls.2025.1641102. eCollection 2025. Front Plant Sci. 2025. PMID: 40772046 Free PMC article. Review.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
