The role of ubiquitination and deubiquitination in urological tumours
- PMID: 40771930
- PMCID: PMC12325273
- DOI: 10.3389/fphar.2025.1532878
The role of ubiquitination and deubiquitination in urological tumours
Abstract
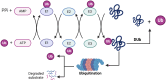
The ubiquitin (Ub) system has been demonstrated to play a crucial role in various cellular processes, including immune responses, cell development, and programmed cell death. Ubiquitination, a form of post-translational modification, occurs in eukaryotic cells and involves several key components, such as Ub-activating enzymes, Ub-binding enzymes, and Ub-protein ligases. Recently, deubiquitinating enzymes-proteases that reverse the modification of proteins by removing Ub or Ub-like molecules, or by remodeling Ub chains on target proteins-have been identified as significant regulators of ubiquitination-mediated degradation. These enzymes profoundly influence cellular pathways and numerous biological processes, including the DNA damage response and DNA repair mechanisms. Recent studies increasingly demonstrate a relationship between ubiquitination, deubiquitination, and urinary diseases. The roles of these processes in urinary diseases are complex, encompassing various aspects of signaling, protein stability, and cellular metabolism. As research advances, the specific mechanisms by which these processes influence urologic diseases will be further clarified. This review examines recent discoveries in this field, aiming to provide new strategies and targets for the diagnosis and treatment of urologic diseases.
Keywords: deubiquitination; molecular mechanisms; targeted therapy; ubiquitination; urinary system.
Copyright © 2025 Gan, Luo, Zou, Li, Chen, Cheng, Zhang, Zhong, Zheng and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Pathological roles of ubiquitination and deubiquitination systems in sepsis-induced myocardial dysfunction.Biomol Biomed. 2025 May 8;25(7):1444-1458. doi: 10.17305/bb.2024.11738. Biomol Biomed. 2025. PMID: 39803908 Free PMC article. Review.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Benjamin D. I., Cozzo A., Ji X., Roberts L. S., Louie S. M., Mulvihill M. M., et al. (2013). Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc. Natl. Acad. Sci. U. S. A. 110, 14912–14917. 10.1073/pnas.1310894110 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

