A comparative study of bone density in elderly people measured with AI and QCT
- PMID: 40771941
- PMCID: PMC12325224
- DOI: 10.3389/frai.2025.1582960
A comparative study of bone density in elderly people measured with AI and QCT
Abstract
Background: Osteoporosis, a systemic skeletal disorder characterized by deteriorated bone microarchitecture and low bone mass, poses substantial fracture risks to aging populations globally. Early detection of reduced bone mineral density (BMD) through opportunistic screening is critical for preventing fragility fractures. Although dual-energy X-ray absorptiometry (DXA) is the gold standard for diagnosing osteoporosis, many patients have not undergone screening with this technique. Therefore, developing an automated tool that can diagnose bone density through routine chest and abdominal CT examinations is highly important. With advancements in technology and the accumulation of clinical data, the role of bone density artificial intelligence (AI) in the diagnosis and management of osteoporosis is becoming increasingly significant.
Objective: First to validate the diagnostic equivalence of AI-based BMD prediction against quantitative CT (QCT) reference standards, second to assess inter-device measurement consistency across multi-vendor CT systems (Siemens, GE, Philips). Ultimately, the objective is to determine the clinical utility of AI-derived BMD for osteoporosis classification.
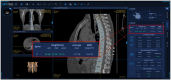
Methods: In this retrospective multicenter study, paired CT/QCT datasets from 702 patients (2019-2022) were analyzed. The accuracy, sensitivity, and specificity of an Bone Density AI model were evaluated by comparing the predicted bone mineral density values from bone density AI with the measured values from QCT. Moreover, the consistency of lumbar spine BMD measurements between QCT and Bone Density AI on different devices was compared.
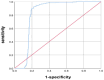
Results: The AUC of Bone Density AI model in diagnosing osteoporosis was 0.822 (95% CI: 0.787-0.867, p < 0.001), with an accuracy of 0.9456, sensitivity of 0.9601, and specificity of 0.9270, indicating good performance in predicting bone density. The consistency study between Bone Density AI and QCT for the vertebral BMD measurements revealed no statistically significant difference in R 2 values, suggesting no significant difference in performance between the two methods in measuring BMD. The linear regression fit between the R 2 values of QCT and Bone Density AI for measuring lumbar spine BMD with different equipment ranged from 0.88 to 0.96, indicating a high degree of consistency between the two measurement methods across devices.
Conclusion: This multicenter study pioneers a dual-validation framework to establish the clinical validity of deep learning-based BMD prediction algorithms using routine thoracic/abdominal CT scans. Our data suggest that AI-driven BMD quantification demonstrates non-inferior diagnostic accuracy to QCT while overcoming DXA's accessibility limitations. This technology enables cost-effective, radiation-free osteoporosis screening through routine CT repurposing, particularly beneficial for resource-constrained settings.
Keywords: BMD values; artificial intelligence; bone density; osteoporosis; quantitative QCT.
Copyright © 2025 Guo, Zhang, Gu, Liu, Peng, Zhang, Jing and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Alternatives to DEXA for the assessment of bone density: a systematic review of the literature and future recommendations.J Neurosurg Spine. 2023 Jan 6;38(4):436-445. doi: 10.3171/2022.11.SPINE22875. Print 2023 Apr 1. J Neurosurg Spine. 2023. PMID: 36609369
-
Quantitative computed tomography provides improved accuracy for diagnosis of lumbar osteoporosis in patients with facet joint osteoarthritis: a cross-sectional study.Osteoporos Int. 2025 Jul 27. doi: 10.1007/s00198-025-07600-3. Online ahead of print. Osteoporos Int. 2025. PMID: 40715756
-
Effectiveness and safety of vitamin D in relation to bone health.Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235. Evid Rep Technol Assess (Full Rep). 2007. PMID: 18088161 Free PMC article.
-
Deep Learning-enhanced Opportunistic Osteoporosis Screening in Ultralow-Voltage (80 kV) Chest CT: A Preliminary Study.Acad Radiol. 2025 Jul;32(7):4254-4265. doi: 10.1016/j.acra.2024.11.062. Epub 2025 May 2. Acad Radiol. 2025. PMID: 40318972
-
Treatment for osteoporosis in people with beta-thalassaemia.Cochrane Database Syst Rev. 2023 May 9;5(5):CD010429. doi: 10.1002/14651858.CD010429.pub3. Cochrane Database Syst Rev. 2023. PMID: 37159055 Free PMC article.
References
-
- Bredella M. A., Daley S. M., Kalra M. K., Brown J. K., Miller K. K., Torriani M. (2015). Marrow adipose tissue quantification of the lumbar spine by using dual-energy CT and single-voxel 1H MR spectroscopy: a feasibility study. Radiology 277, 230–235. doi: 10.1148/radiol.2015142876, PMID: - DOI - PMC - PubMed
-
- Chen L., Pan Y., Zhong F., Yuan T. J., Wang H., Chen T., et al. (2022). Using QCT to evaluate bone mineral and abdominal adipose changes in patients with primary hyperparathyroidism and comparing it to DXA for bone status assessment: a retrospective case-control study. Ann. Transl. Med. 10:606. doi: 10.21037/atm-22-1827, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

