Copper-based nanoparticles for the removal of the crystal violet dye via degradation and adsorption: a comparative account
- PMID: 40772009
- PMCID: PMC12327229
- DOI: 10.1039/d5ra04003e
Copper-based nanoparticles for the removal of the crystal violet dye via degradation and adsorption: a comparative account
Abstract
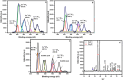
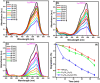
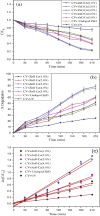
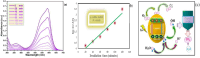
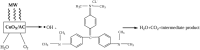
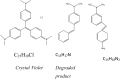
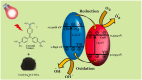
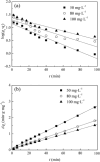
Nanoparticles are almost omnipresent with significant pros and cons. Scientists have ventured into environmental nanotechnology, synthesizing unique nanoparticles in the laboratory. Copper nanoparticles are an active area of research for the decontamination of water from toxic dyes in the context of environmental nanotechnology. Copper is an abundant element in our earth's crust. However, rapid aerial oxidation limits its application. The present review article focuses on removing a toxic dye crystal violet (CV) via adsorption and degradation involving copper-based nanoparticles. Various synthetic protocols of such nanoparticles, removal efficiency including reusability, effect of doping and physico-chemical conditions, mechanisms, and connections towards circular economy were summarized here. A comparative account was depicted between adsorption and degradation for the elimination of CV involving copper-based nanoparticles. The current review paper will hopefully be an asset for the industries that release harmful dyes for sustainable water management.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no competing interest to disclose.
Figures






Similar articles
-
Biofabrication of zinc oxide nanoparticles using Moringa oleifera, characterization and statistical optimization for their application in crystal violet adsorption.Sci Rep. 2025 Jan 30;15(1):3780. doi: 10.1038/s41598-025-86629-0. Sci Rep. 2025. PMID: 39885265 Free PMC article.
-
Application of the synergism between eggshells and copper in nanotechnology.Nanoscale Adv. 2025 Apr 30;7(13):3914-3940. doi: 10.1039/d5na00400d. eCollection 2025 Jun 24. Nanoscale Adv. 2025. PMID: 40417167 Free PMC article. Review.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
References
-
- Ighalo J. O. Sagboye P. A. Umenweke G. Ajala O. J. Omoarukhe F. O. Adeyanju C. A. Ogunniyi S. Adeniyi A. G. Environ. Nanotechnol. Monit. Manag. 2021;15:100443.
-
- Narayanan M. Salmen S. H. Chinnathambi A. Suresh K. Ramesh Barathi S. Lee J. J. Taiwan Inst. Chem. Eng. 2025;166:105258.
-
- Lata S. Siddharth Energy Nexus. 2021;3:100020.
-
- Aragaw T. A. J. Hazard. Mater. Adv. 2024;16:100493.
-
- Lellis B. Fávaro-Polonio C. Z. Pamphile J. A. Polonio J. C. Biotechnol. Res. Innov. 2019;3:275–290.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

