The PEAPOD repressor complex in Arabidopsis stomatal development
- PMID: 40772046
- PMCID: PMC12325319
- DOI: 10.3389/fpls.2025.1641102
The PEAPOD repressor complex in Arabidopsis stomatal development
Abstract
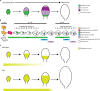
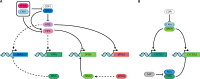
Stomata comprise two guard cells that function as microscopic valves in the plant epidermis, connecting mesophyll interstices to the atmosphere. Stomata regulate gas exchange and evapotranspiration, directly impacting photosynthesis and leaf temperature regulation, and their function is thus crucial for plant adaptability and fitness. In Arabidopsis, stomatal development is primarily driven by three basic helix-loop-helix transcription factors: SPEECHLESS (SPCH), MUTE, and FAMA, and occurs within the broader context of leaf development. During leaf development, a characteristic division-to-differentiation transition zone, marked by the first cell cycle arrest front (1st AF), progresses from the apex to the base of the leaf blade. The repeated division of meristemoids (M), self-renewing cells of stomatal lineages, is not halted during 1st AF, requiring a second arrest front, which is associated with activity of the PEAPOD (PPD) proteins, PEAPOD1 (PPD1) and PEAPOD2 (PPD2), which form a transcriptional repressor complex that halts M stem cell-like activity; however, the relationship between PPDs and stomatal development has not been fully elucidated. Here, we review data on PPD-mediated regulation of light signaling and the cell cycle and the influence of these factors on stomatal development.
Keywords: PEAPOD; asymmetric cell division; cell cycle; leaf development; light signaling; stomatal development.
Copyright © 2025 Saiz-Pérez, Fenoll and Mena.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adrian J., Chang J., Ballenger C. E., Bargmann B. O. R., Alassimone J., Davies K. A., et al. (2015). Transcriptome dynamics of the stomatal lineage: birth, amplification, and termination of a self-renewing population. Dev. Cell 33, 107–118. doi: 10.1016/j.devcel.2015.01.025, PMID: - DOI - PMC - PubMed
-
- Asl L. K., Dhondt S., Boudolf V., Beemster G. T. S., Beeckman T., Inzé D., et al. (2011). Model-based analysis of Arabidopsis leaf epidermal cells reveals distinct division and expansion patterns for pavement and guard cells. Plant Physiol. 156, 2172–2183. doi: 10.1104/pp.111.181180, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

