Role of Intravenous Azithromycin as Adjunctive Therapy in Children With Acute Encephalitis Syndrome (AES): An Open-Label Randomized Controlled Trial
- PMID: 40772165
- PMCID: PMC12324931
- DOI: 10.7759/cureus.87387
Role of Intravenous Azithromycin as Adjunctive Therapy in Children With Acute Encephalitis Syndrome (AES): An Open-Label Randomized Controlled Trial
Abstract
Background: Acute encephalitis syndrome (AES) has high morbidity and mortality in children. Empirical treatment of AES often consists of third-generation cephalosporins, with vancomycin and acyclovir, and frequently excludes the use of azithromycin, targeted at scrub typhus. In a resource-constrained setting, testing for scrub typhus becomes challenging. Considering the lacunae in the existing literature, there is a need for robust evidence to determine the role of additional azithromycin use in children with AES. Objective: To evaluate the efficacy and safety of adjunctive intravenous azithromycin treatment in children with AES compared to standard therapy alone.
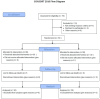
Material and methods: An open-label, two-arm randomized controlled trial was conducted at a tertiary care teaching hospital with a level III pediatric intensive care unit. Children aged one to 14 years with AES were enrolled. Intravenous azithromycin in addition to conventional treatment (n=30) and conventional treatment (third-generation cephalosporin, vancomycin, and acyclovir) were compared. The primary outcome measure was all-cause mortality. Secondary outcome measures included the total length of hospital stay, the proportion of children with significant disability as determined by the Liverpool Outcome Score (LOS) at discharge, and the proportion of children who experienced at least one serious adverse event.
Results: The two groups were comparable in terms of all-cause mortality (23.3% vs. 20%; p=0.75), duration of hospital stay (12.57 days vs. 13.67 days; P=0.28), and significant disability at the time of discharge (52.40 vs. 53.47 days; P=0.79). None developed serious life-threatening adverse events.
Conclusion: Additional treatment with intravenous azithromycin does not impact all-cause mortality among children with AES. Further evaluation is suggested with an adequately powered study and long-term follow-up.
Keywords: acute encephalitis; azithromycin; cerebrospinal fluid; iv ceftriaxone; pediatric scrub typhus.
Copyright © 2025, Yadav et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Biomedical Research Ethics Committee of Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences issued approval IEC/Th/19/Ped04. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Antibiotics for exacerbations of asthma.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD002741. doi: 10.1002/14651858.CD002741.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938789 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
-
Antibiotics for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Oct 22;10(10):CD015025. doi: 10.1002/14651858.CD015025. Cochrane Database Syst Rev. 2021. PMID: 34679203 Free PMC article.
References
-
- Consensus guidelines on evaluation and management of suspected acute viral encephalitis in children in India. Sharma S, Mishra D, Aneja S, Kumar R, Jain A, Vashishtha VM. Indian Pediatr. 2012;49:897–910. - PubMed
-
- Changing landscape of acute encephalitis syndrome in India: a systematic review. Joshi R, Kalantri SP, Reingold A, Colford JM Jr. https://pubmed.ncbi.nlm.nih.gov/23278779/ Natl Med J India. 2012;25:212–220. - PubMed
LinkOut - more resources
Full Text Sources