Effectiveness and Safety of Switching to Fixed-Dose, Preservative-Free Tafluprost/Timolol in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: A Real-World Study in Taiwan
- PMID: 40772200
- PMCID: PMC12327549
- DOI: 10.7759/cureus.87447
Effectiveness and Safety of Switching to Fixed-Dose, Preservative-Free Tafluprost/Timolol in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: A Real-World Study in Taiwan
Abstract
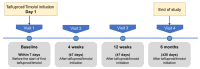
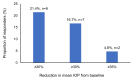
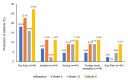
Objective The objective of this study is to evaluate the effectiveness, safety, and tolerability of a preservative-free fixed-dose combination of tafluprost (0.0015%) and timolol (0.5%) (PF tafluprost/timolol FC) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT). Methodology This real-world, prospective, noninterventional study was conducted in Taiwanese patients with OAG or OHT who had an inadequate response or intolerance to topical prostaglandin analogue (PGA) monotherapy and were therefore switched to PF tafluprost/timolol FC. The primary endpoint was the mean change in intraocular pressure (IOP) from baseline to six months after initiating treatment. Changes in clinical signs and subjective symptoms were also evaluated, and adverse events (AEs) were recorded. Results A total of 49 patients were enrolled, and 42 completed the study. The mean ± SD IOP at baseline was 16.5 ± 3.5 mmHg, which significantly decreased to 15.4 ± 3.4 mmHg at six months (absolute reduction: 1.1 ± 2.6 mmHg; p < 0.001). The number of patients with a tear break-up time >10 seconds increased significantly from three (7.5%) at baseline to 22 (52.5%) at six months (p < 0.001). However, a nonsignificant increase was observed in subjective symptoms. Six treatment-related AEs were reported, all of which were nonserious and mild to moderate in severity, including contact dermatitis, redness and itchiness, and blurred vision. Conclusions This real-world, prospective study in Taiwan demonstrated that switching patients with OAG or OHT from PGA monotherapy to PF tafluprost/timolol FC was effective and safe for reducing IOP.
Keywords: fixed-dose combination; glaucoma; ocular hypertension; preservative-free; tafluprost.
Copyright © 2025, Su et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Chang Gung Medical Foundation Institutional Review Board issued approval 202002011A3C501. The study was performed following the current version of the Declaration of Helsinki in agreement with the International Conference on Harmonisation Guidelines on Good Clinical Practice. The study was registered with ClinicalTrials.gov (NCT04828057; registration date: April 1, 2021) and performed in compliance with the requirements of the Linkou Chang Gung Memorial Hospital Medical Center Institutional Review Board. The protocol and informed consent form were approved by the Institutional Review Board on December 24, 2020; revisions were approved on August 21, 2021 (case no. 202002011A3C501). Patient enrollments were started on September 1, 2021, and all patients provided written informed consent to participate in the study prior to study screening activities. Principal Investigator(s): Wei-Wen-Su; Co-Investigator(s): Yung-Sung-Lee; Study Coordinator(s): Shu-hui Yang; Executing Institution: Linkou; Duration of Approval: From 2021/8/21 To 2021/12/15; Date of Approval: 2021/08/21; Frequency of Continuing Report: Once a year; Next Deadline of Continuing Report: 2021/12/15. To facilitate the review, please submit the report two months before the deadline (or one month before the expiration of the trial if a continuing report shall be provided every three months) in order not to influence the principal investigator’s right to conduct the research. In the case that failure or delay to submit a continuing report makes the IRB unable to determine the next trial period by the deadline, the trial shall not be continuously conducted. If the Principal Investigator fails to submit a continuing report on time, rendering the Institutional Review Board unable to issue the next trial execution period before the previous trial execution period expires, all research activities, including the intervention or interaction with the participating trial subjects, must be suspended. Unless the Institutional Review Board considers that the continuation of trial intervention or trial participation is greatly beneficial to the trial subject's safety or in the best interest of the trial subject from a moral point of view, no new trial subject shall be included until the continuing report is formally approved. The IRB is organized and operates in accordance with Good Clinical Practice and the applicable laws and regulations. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures



Similar articles
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
Treatment of Open-Angle Glaucoma and Ocular Hypertension with the Fixed-Dose Combination of Preservative-Free Tafluprost/Timolol: Clinical Outcomes from Ophthalmology Clinics in Italy.Clin Ophthalmol. 2022 Jun 1;16:1707-1719. doi: 10.2147/OPTH.S364880. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35677639 Free PMC article.
-
Switching to Preservative-Free Tafluprost/Timolol Fixed-Dose Combination in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: Subanalysis of Data from the VISIONARY Study According to Baseline Monotherapy Treatment.Adv Ther. 2022 Aug;39(8):3501-3521. doi: 10.1007/s12325-022-02166-6. Epub 2022 May 7. Adv Ther. 2022. PMID: 35524840 Free PMC article.
-
A Study of 24-h Efficacy and Safety of Sepetaprost vs. Latanoprost in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension.Adv Ther. 2025 Aug;42(8):3810-3825. doi: 10.1007/s12325-025-03227-2. Epub 2025 Jun 10. Adv Ther. 2025. PMID: 40493333 Free PMC article. Clinical Trial.
-
Laser trabeculoplasty for open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD003919. doi: 10.1002/14651858.CD003919.pub3. Cochrane Database Syst Rev. 2022. PMID: 35943114 Free PMC article.
References
-
- Glaucoma. Gupta D, Chen PP. https://www.aafp.org/pubs/afp/issues/2016/0415/p668.html. Am Fam Physician. 2016;93:668–674. - PubMed
LinkOut - more resources
Full Text Sources
