Prostaglandin E2 and Progesterone Receptor Coordinately Regulate Primary Cilia for Proper Decidualization
- PMID: 40772309
- PMCID: PMC12329624
- DOI: 10.1096/fj.202500961RR
Prostaglandin E2 and Progesterone Receptor Coordinately Regulate Primary Cilia for Proper Decidualization
Abstract
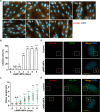
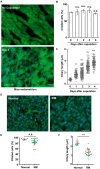
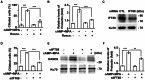
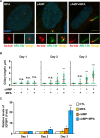
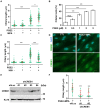
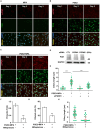
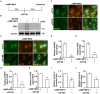
Decidualization, the process by which endometrial stromal cells differentiate into decidual cells in response to prostaglandin E2 (PGE2) and progesterone receptor (PGR) signaling, is essential for proper implantation and placentation. Primary cilia, microtubule-based cellular antennae, contribute to various differentiation processes, including decidualization. In this study, we demonstrated that both the proportion of ciliated cells and ciliary length increased in a time-dependent manner during in vitro decidualization. In a mouse model, after copulation, the proportion of ciliated cells fluctuated, but ciliary length progressively increased over time. Additionally, we observed defective primary cilia in the endometrium of women with recurrent miscarriages. Mechanistically, we found that primary cilia were present before the expression of decidual markers under decidual stimulation. Depletion or inhibition of primary cilia impaired decidualization, highlighting their critical role in this process. Furthermore, we identified the PGE2-PKA-CREB1 axis as a key regulator of ciliary growth and PGR upregulation. Upon progesterone stimulation, active PGR further increased ciliary length, thereby facilitating decidualization. Thus, our study not only establishes a link between ciliary length and decidualization but also elucidates the sequential regulation of ciliary dynamics by PGE2 and PGR in a coordinated manner.
Keywords: cAMP; decidualization; primary cilium; progesterone receptor; prostaglandin E2.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
NR5A1 Deficiency Leads to Decidualization Dysregulation of Stromal Cells and Recurrent Spontaneous Abortion During Early Pregnancy.FASEB J. 2025 Jun 30;39(12):e70752. doi: 10.1096/fj.202500781R. FASEB J. 2025. PMID: 40542600
-
The Long Non-Coding RNA Gene AC027288.3 Plays a Role in Human Endometrial Stromal Fibroblast Decidualization.Cells. 2024 May 2;13(9):778. doi: 10.3390/cells13090778. Cells. 2024. PMID: 38727314 Free PMC article.
-
EHD1 impaired decidualization of endometrial stromal cells in recurrent implantation failure: role of SENP1 in modulating progesterone receptor signalling†.Biol Reprod. 2024 Mar 13;110(3):536-547. doi: 10.1093/biolre/ioad161. Biol Reprod. 2024. PMID: 38011671
-
Progestogens for preventing miscarriage: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD013792. doi: 10.1002/14651858.CD013792.pub2. Cochrane Database Syst Rev. 2021. PMID: 33872382 Free PMC article.
-
Luteal phase support for assisted reproduction cycles.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD009154. doi: 10.1002/14651858.CD009154.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jul 07;(7):CD009154. doi: 10.1002/14651858.CD009154.pub3. PMID: 21975790 Updated.
References
-
- Chao Y. Y., Huang B. M., Peng I. C., et al., “ATM‐ and ATR‐Induced Primary Ciliogenesis Promotes Cisplatin Resistance in Pancreatic Ductal Adenocarcinoma,” Journal of Cellular Physiology 237 (2022): 4487–4503. - PubMed
-
- Lin R. C., Chao Y. Y., Su M. T., Tsai H. L., Tsai P. Y., and Wang C. Y., “Upregulation of miR‐20b‐5p Inhibits Trophoblast Invasion by Blocking Autophagy in Recurrent Miscarriage,” Cellular Signalling 113 (2024): 110934. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

