Hinge Truncation to Improve Aggregation Kinetics and Thermal Stability of an Antibody Fab Fragment
- PMID: 40772589
- PMCID: PMC12406249
- DOI: 10.1021/acs.molpharmaceut.5c00358
Hinge Truncation to Improve Aggregation Kinetics and Thermal Stability of an Antibody Fab Fragment
Abstract
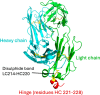
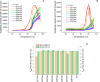
The hinge region of antibody fragments plays a crucial role in their stability and aggregation properties. In this study, we investigated the effects of hinge truncations on the thermal stability and aggregation propensity of the A33 Fab antibody fragment. Eight Fab variants were engineered by introducing stop codons to truncate 1-8 residues at the hinge region (heavy chain residues 221-228). These variants were then expressed, purified, and characterized in terms of stability and aggregation propensity using SDS-PAGE, SEC-HPLC, LC-MS, and thermal stability assays. Our findings demonstrate that truncating the hinge region can enhance the thermal stability and reduce the aggregation of Fab fragments, and that progressive truncations identified an optimal hinge length for stability. Notably, the 227TGA variant exhibited a significant 14.5% reduction in aggregation rate compared to the wild type, without compromising thermal stability. By contrast, 221TGA removed all of the hinge and reduced the aggregation rate by 13%, but also decreased the thermal stability. These results suggest that hinge truncation is a promising strategy for improving the developability of therapeutic antibody Fab fragments by mitigating some of the stability issues associated with aggregation.
Keywords: aggregation; engineering; hinge; stability; therapeutic; truncation.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

