Bicyclo[1.1.0]tetragermane-2,4-diide Diradicaloid
- PMID: 40772647
- PMCID: PMC12435412
- DOI: 10.1002/anie.202513772
Bicyclo[1.1.0]tetragermane-2,4-diide Diradicaloid
Abstract
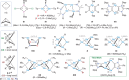
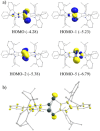
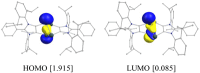
The synthesis, structure, and reactivity of the bicyclo[1.1.0]tetragermane-2,4-diide compound [(ADC)Ge2]2 (3), which features a Ge4 core bridged by two anionic dicarbene frameworks (ADC = PhC{N(Dipp)C}2; Dipp = 2,6-iPr2C6H3), are reported. Treatment of an alkyne-functionalized amidine Me3SiC≡CN(Dipp)C(Ph)═N(Dipp) (1) with GeCl4 affords [(ADC)GeCl3(GeCl4)] (2). KC8 reduction of 2 yields 3 as a Venetian red crystalline solid. DFT calculations reveal a singlet ground state for 3 with the singlet-triplet energy gap of 14 kcal mol-1. CASSCF (complete active space self-consistent field) calculations suggest a modest diradical character (β = 9%) for 3. Compound 3 readily reacts with TEMPO (2,2,6,6-tetramethylpiperidinyloxyl) to yield the Ge─Ge bond-cleaved product, [(ADC)Ge(Ge-TEMPO)]2 (4). Treatment of 3 with Fe2(CO)9 gives [(ADC)Ge(Ge{Fe(CO)4})]2 (5).
Keywords: Carbene; Cluster; Diradicaloid; Germanium; Main group; Stretched bond.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Stuyver T., Chen B., Zeng T., Geerlings P., De Proft F., Hoffmann R., Chem. Rev. 2019, 119, 11291–11351. - PubMed
-
- Abe M., Chem. Rev. 2013, 113, 7011–7088. - PubMed
-
- Salem L., Rowland C., Angew. Chem. Int. Ed. 1972, 11, 92–111.
-
- Lee C. K., Gangadharappa C., Fahrenbach A. C., Kim D. J., Adv. Mat. 2024, 36, 2408271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

