Artesunate Treats Aspergillus fumigatus Keratitis by Inhibiting Fungal Activity and Activating Autophagy Pathway to Reduce Corneal Inflammation
- PMID: 40772662
- PMCID: PMC12347252
- DOI: 10.1167/iovs.66.11.19
Artesunate Treats Aspergillus fumigatus Keratitis by Inhibiting Fungal Activity and Activating Autophagy Pathway to Reduce Corneal Inflammation
Abstract
Purpose: The purpose of this study was to investigate the therapeutic effect of artesunate (ART) on fungal keratitis (FK).
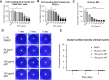
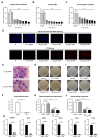
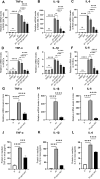
Methods: The antifungal properties of ART against Aspergillus fumigatus (A. fumigatus) were confirmed by minimum inhibitory concentration (MIC), biofilm formation inhibition test, propidium iodide fluorescence, and calcium fluorescence white test. The levels of HSP90, BrlA, AbaA, WetA, CnaA, and CrzA were detected by quantitative reverse transcription PCR (qRT-PCR). Cell counting kit 8 (CCK-8) was used to detect the cytotoxicity of ART on RAW 264.7 cells and human corneal epithelial cells (HCECs). Clinical corneal score, hematoxylin and eosin (H&E) staining, and corneal fungal plate counts were used to evaluate the therapeutic effect of ART on FK in mice. The qRT-PCR, ELISA, and Western blot were used to investigate the effect of ART on inflammatory mediator expression during fungal infection.
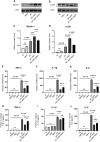
Results: ART inhibited the growth of A fumigatus, biofilm formation, and conidium adhesion in vitro, and destroyed fungal cell walls and cytomembrane. In vivo, ART effectively reduced corneal fungal load. ART could reduce the inflammation of the cornea by reducing the accumulation of inflammatory cells and down-regulating the expression of TNF-α, IL-1β, and IL-6. ART could activate the autophagy pathway to play an anti-inflammatory role in FK by increasing the expression of Beclin-1 and LC3B.
Conclusions: ART inhibits fungal growth by inhibiting biofilm formation, destroying fungal cell walls and membranes, and reducing the expression of HSP90 and calcineurin-related factors, and activates the autophagy pathway to reduce the expression of TNF-α, IL-1β, and IL-6 in FK by upregulating the protein expression of Beclin-1 and LC3B. ART has therapeutic potential for FK.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Baicalein Protects Against Aspergillus fumigatus Keratitis by Reducing Fungal Load and Inhibiting TSLP-Induced Inflammatory Response.Invest Ophthalmol Vis Sci. 2021 May 3;62(6):26. doi: 10.1167/iovs.62.6.26. Invest Ophthalmol Vis Sci. 2021. PMID: 34038512 Free PMC article.
-
Isorhamnetin Ameliorates Aspergillus fumigatus Keratitis by Reducing Fungal Load, Inhibiting Pattern-Recognition Receptors and Inflammatory Cytokines.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):38. doi: 10.1167/iovs.62.3.38. Invest Ophthalmol Vis Sci. 2021. PMID: 33783487 Free PMC article.
-
Zeolitic Imidazolate Framework-8 Offers an Anti-Inflammatory and Antifungal Method in the Treatment of Aspergillus Fungal Keratitis in vitro and in vivo.Int J Nanomedicine. 2024 Nov 1;19:11163-11179. doi: 10.2147/IJN.S480800. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39502641 Free PMC article.
-
Medical interventions for fungal keratitis.Cochrane Database Syst Rev. 2015 Apr 9;2015(4):CD004241. doi: 10.1002/14651858.CD004241.pub4. Cochrane Database Syst Rev. 2015. PMID: 25855311 Free PMC article.
-
Emerging ocular pathogen: multidrug-resistant Corynespora cassiicola in fungal keratitis: case series and literature review.Int Ophthalmol. 2025 Jul 2;45(1):274. doi: 10.1007/s10792-025-03637-9. Int Ophthalmol. 2025. PMID: 40601186 Review.
References
-
- Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021; 21(3): e49–e57. - PubMed
-
- Liu MY, Zhang L, Yin XL, Sun SY. Endophthalmitis associated with fungal keratitis and penetrating injuries in North China. Eur J Ophthalmol. 2020; 30(3): 455–461. - PubMed
-
- Yu B, Li C, Gu L, et al.. Eugenol protects against Aspergillus fumigatus keratitis by inhibiting inflammatory response and reducing fungal load. Eur J Pharmacol. 2022; 924: 174955. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

